Health Canada Medical Device Fee Form Title Medical Device Application Fee Form Author Health Canada Government of Canada Sant Canada Gouvernement du Canada Created Date 2 26 2020 2 11 01 PM
Medical Device Licence Application Fee Form 2023 04 01 Application For a New Class IV Medical Device Licence Updated PDF fillable saveable 721 K 2022 Category Fee Class II New licence application 414 Class III New licence application 5 922 Class III New licence application for a near patient in vitro diagnostic device
Health Canada Medical Device Fee Form

Health Canada Medical Device Fee Form
https://jpiko.com/blogimgs/https/cip/jobsalert.pk/wp-content/uploads/2017/11/ISB.gif

Health Canada Guidance On Applying For An MDEL RegDesk
https://www.regdesk.co/wp-content/uploads/2021/06/Health-Canada-1-980x656-1.jpg

How To Get Medical Device License In Canada Medical Device Sales In
https://i.ytimg.com/vi/S3r_cMfPWTw/maxresdefault.jpg
Invoicing and fee payment Medical device licences Manufacturers wanting to obtain a medical device licence must complete a Medical Devices Application Fee Form Medical Devices Directorate Health Canada 11 Holland Avenue Address Locator 3002A OTTAWA Ontario K1A 0K9 All application types will be validated for administrative
Health Canada issues two different types of licences for medical devices Medical Device Licence MDL a licence issued to manufacturers authorizing them to import or sell Fees for the Review of Medical Device Licence Applications Date adopted 1997 05 01 Date posted 2019 11 04 Effective date 2020 04 01 Health Canada is responsible for helping
Download Health Canada Medical Device Fee Form
More picture related to Health Canada Medical Device Fee Form

Canada s Government Run Health Care System Crumbled Under COVID 19
https://fee.org/media/37380/canadian-doctor.jpg?anchor=center&mode=crop&width=1800&rnd=132333210310000000

Certificate Health Canada By DESS USA Issuu
https://image.isu.pub/170825185307-ca13a6b15a8811750b198417477f2d36/jpg/page_1.jpg
Health Canada Launches Consultations Fee Proposal For Drugs And
https://media.licdn.com/dms/image/C5612AQHBF9Tk3rvNtg/article-cover_image-shrink_600_2000/0/1520146550333?e=2147483647&v=beta&t=B_vc6hOS9dPxco8HnPiD1Dp3wbUGInupXYHqxT4Lokk
The renewal process has two purposes the first is to confirm whether the medical device will continue to be sold in Canada and the medical device licence will remain active the To increase predictability and transparency for industry Health Canada has drafted a new policy on medical device establishment licence terms and conditions
Canada Health Canada issuing body Guidance on how to complete the application for a new medical device licence View H164 315 2021 eng pdf PDF 940 KB View Compared to 2021 there has also been an increase in the fee for the examination of an application Medical Device Establishment Licence that as from 1 st

Health Canada Medical Device Reporting Database
https://learn.marsdd.com/wp-content/uploads/2022/02/data-requirements-risk-1024x538.jpg

Health Canada s Special Access Program For Medical Devices
https://emmainternational.com/wp-content/uploads/2020/12/HealthCanadaSpecial_Blog-01-1024x683.jpg

https://www.canada.ca/content/dam/hc-sc/documents/...
Title Medical Device Application Fee Form Author Health Canada Government of Canada Sant Canada Gouvernement du Canada Created Date 2 26 2020 2 11 01 PM

https://www.canada.ca/en/health-canada/services/...
Medical Device Licence Application Fee Form 2023 04 01 Application For a New Class IV Medical Device Licence Updated PDF fillable saveable 721 K 2022

Health Canada Guidance On License Application Types RegDesk

Health Canada Medical Device Reporting Database
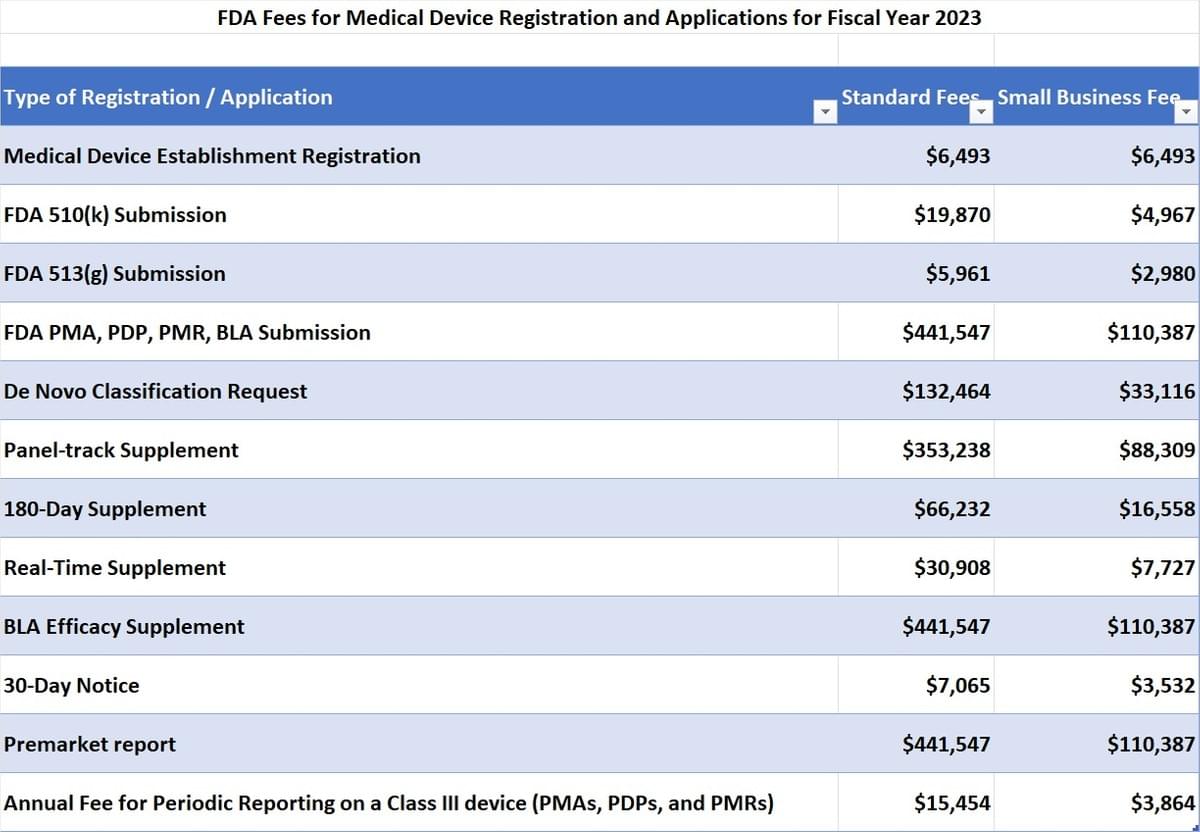
Medical Device User Fee Rates For Fiscal Year 2023

CANADIAN MEDICAL DEVICE REGULATIONS SOR 98 282 PDF

Health Canada Medical Device Regulations Help Health
Understanding Medical Device User Fees

Understanding Medical Device User Fees

Late Payment Fee Doc Template PdfFiller

Recent Changes To Medical Device Regulations In Canada RegDesk

Is Health Care Really Free In Canada University Magazine
Health Canada Medical Device Fee Form - Health Canada issues two different types of licences for medical devices Medical Device Licence MDL a licence issued to manufacturers authorizing them to import or sell
