Ich Gcp Guidelines 2021 Pdf 9 The objective of this ICH GCP Guideline is to provide a unified standard to facilitate the mutual 10 acceptance of clinical trial data for ICH member countries and regions by applicable regulatory 11 authorities 12 This guideline builds on key concepts outlined in ICH E8 R1 General Considerations for 13 Clinical Studies
The objective of this ICH GCP Guideline is to provide a unified standard for the European Union EU Japan and the United States to facilitate the mutual acceptance of clinical data by the regulatory authorities in these jurisdictions Guideline for good clinical practice E6 R2 EMA CHMP ICH 135 1995 Page 7 75 159 Introduction 160 Good Clinical Practice GCP is an international ethical and scientific quality standard for 161 designing conducting recording and reporting trials that involve the participation of human subjects
Ich Gcp Guidelines 2021 Pdf

Ich Gcp Guidelines 2021 Pdf
https://i.ytimg.com/vi/TCW55krWzb4/maxresdefault.jpg

Virtual Chat On Call Dentist And 13 Core Principles Of ICH GCP Blog
http://www.virtualdentist.in/blog/wp-content/uploads/2020/08/1-2.png
The Importance Of ICH GCP CCRPS
http://static1.squarespace.com/static/56a042fb25981d9326c9bbdb/t/6091584d9efd40482ae228c5/1620138074539/ich+gcp?format=1500w
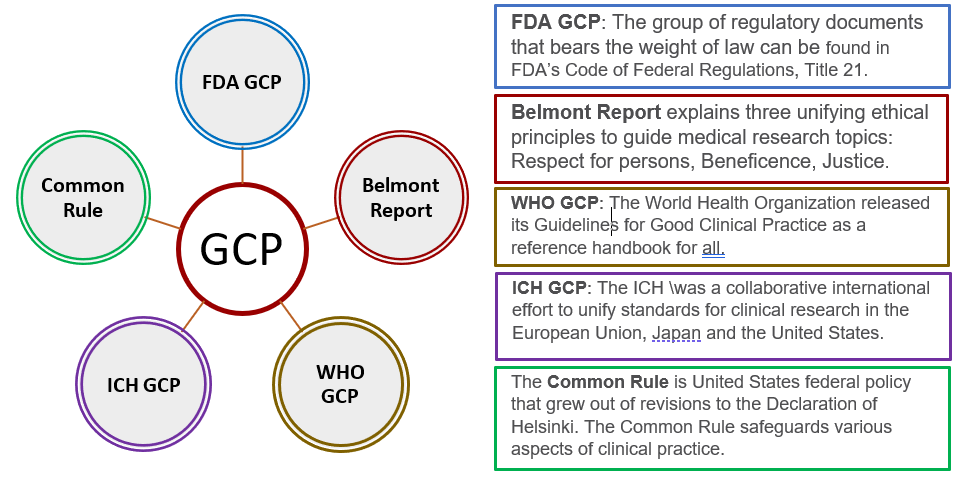
This document addresses the good clinical practice an international ethical and scientific quality standard for designing conducting recording and reporting trials that involve the participation of human subjects The objective of this ICH GCP guidance is to provide a unified standard for the European Union Japan and the United States to facilitate the mutual acceptance of clinical data by the
The ICH E6 Good Clinical Practice GCP Guideline is widely used by clinical trial researchers beyond the membership and regional representation of ICH itself and has a significant impact on trial participants and patients VICH GL9 Good Clinical Practice PDF 64KB The objective of this document is to provide guidance on the design and conduct of all clinical studies of veterinary products in the target
Download Ich Gcp Guidelines 2021 Pdf
More picture related to Ich Gcp Guidelines 2021 Pdf

Barnett Gcp Questions And Answers Clinical Research Certification I
https://i.pinimg.com/736x/67/ce/7a/67ce7a2307c78708afcbdfe200005cd6.jpg

ICH GCP Certificate Nhvm NIDA Clinical Trials Network Certificate
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/a87f4371c3e56c3e0e3972c947ae330e/thumb_1200_1553.png
Fundamentals Of Laboratory Management OER Commons
https://www.oercommons.org/editor/images/40720
Principles of ICH GCP Designed to be flexible and applicable to a broad range of Clinical Trials CTs Annex 1 Considerations for interventional CTs ICH E6 R3 GCP Principles The use of technology in the conduct of clinical trials should be adapted to fit the participant characteristics and the trial design The use of innovative technologies may help enable those designing and conducting a trial to include relevant patient populations
[desc-10] [desc-11]

Good Clinical Practice And ICH GCP Guidelines YouTube
https://i.ytimg.com/vi/Rm6Mcyx7m-Y/maxresdefault.jpg

Good Clinical Practices Guideline ICH GCP Principles Of GCP Hindi
https://i.ytimg.com/vi/JW7ieXoAfGg/maxresdefault.jpg

https://database.ich.org › sites › default › files
9 The objective of this ICH GCP Guideline is to provide a unified standard to facilitate the mutual 10 acceptance of clinical trial data for ICH member countries and regions by applicable regulatory 11 authorities 12 This guideline builds on key concepts outlined in ICH E8 R1 General Considerations for 13 Clinical Studies

https://database.ich.org › sites › default › files
The objective of this ICH GCP Guideline is to provide a unified standard for the European Union EU Japan and the United States to facilitate the mutual acceptance of clinical data by the regulatory authorities in these jurisdictions

GCP Resource Hierarchy Roles And Identities GCP Certification Cheat

Good Clinical Practice And ICH GCP Guidelines YouTube

ICH GCP Guidelines
ICH GCP Guidelines E6 R2 Part 2 On Vimeo

A Primary Purpose Of The Ich E6 Guideline Is To

Good Clinical Practice GCP Guidelines For Clinical Trials By Priya

Good Clinical Practice GCP Guidelines For Clinical Trials By Priya

ICH GCP Guidelines

PDF ANALYSIS OF CLINICAL TRIALS INFORMED CONSENT COMPLIANCE WITH

Monitoring Procedures Workstream Document PDF 379 02 KB
Ich Gcp Guidelines 2021 Pdf - This document addresses the good clinical practice an international ethical and scientific quality standard for designing conducting recording and reporting trials that involve the participation of human subjects