Medicaid Drug Rebate Agreement Web 11 ao 251 t 2022 nbsp 0183 32 The program requires a drug manufacturer to enter into and have in effect a National Drug Rebate Agreement NDRA with the Secretary of the Department of Health and Human Services HHS in exchange for state Medicaid coverage of most of the
Web 31 d 233 c 2020 nbsp 0183 32 Medicaid beneficiaries The MDRP provides specific requirements for rebate agreements drug pricing submission and confidentiality requirements the formulas for calculating rebate payments drug utilization reviews DUR and requirements for states Web 5 sept 2023 nbsp 0183 32 Medicaid Drug Rebate Program Consumer Price Index Urban Value Dispute Resolution File Formats and Data Definitions Interest Calculation for Late Rebate Payments Medicaid Drug Programs MDP System Access Medicaid Drug Rebate
Medicaid Drug Rebate Agreement

Medicaid Drug Rebate Agreement
https://s3.amazonaws.com/images.federalregister.gov/EN09NO16.008/original.png?1478266588

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN09NO16.006/original.png?1481698007

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN23MR18.002/original.png?1521650112
Web 10 f 233 vr 2023 nbsp 0183 32 The statements included on this web page are intended to provide information on Medicaid Drug Rebate Program Releases and do not in any way revise or modify the requirements set forth in Section 1927 of the Act the national drug rebate Web 8 janv 2021 nbsp 0183 32 The final rule defines VBP as an arrangement or agreement intended to align pricing and or payments to an observed or expected therapeutic or clinical value in a select population and includes but is not limited to 1 Evidence based measures which
Web We study how Medicaid drug procurement rules affect private markets leveraging new data and changes to rules implemented in the Affordable Care Act ACA Using a stylized model we illustrate how the rules of the Medicaid Drug Rebate Program MDRP Web Under the Medicaid Drug Rebate Program a drug manufacturer must enter into a Medicaid national drug rebate agreement with the Secretary of the U S Department of Health and Human Services in order for states to receive federal funding for using the
Download Medicaid Drug Rebate Agreement
More picture related to Medicaid Drug Rebate Agreement

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN23MR18.008/original.png?1521650113

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN23MR18.000/original.png?1521650111

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN23MR18.006/original.png?1521650112
Web 21 juil 2022 nbsp 0183 32 These arrangements allow state Medicaid agencies to receive additional discounts or rebates from manufacturers on a drug when a drug does not meet certain observed or expected therapeutic or clinical values in a select population based on Web 1 sept 2023 nbsp 0183 32 Medicaid Drug Rebate Program Consumer Price Index Urban Value Dispute Resolution File Formats and Data Definitions Interest Calculation for Late Rebate Payments Medicaid Drug Programs MDP System Access Medicaid Drug Rebate
Web Medicaid Drug Rebate Program MDRP file formats are used by CMS states and manufacturers to transmit and receive various data required by the MDRP CMS implemented a new Medicaid Drug Programs MDP system in 2021 We have posted Web 19 juin 2020 nbsp 0183 32 Medicaid Drug Rebate program MDRP and payment for covered outpatient drugs CODs which are defined in section 1927 k 2 of the Act In general for payment to be made available for CODs under section 1903 a of the Act manufacturers must enter

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN23MR18.009/original.png?1521650113

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN09NO16.011/original.png?1481698020

https://www.medicaid.gov/.../medicaid-drug-rebate-program/index.html
Web 11 ao 251 t 2022 nbsp 0183 32 The program requires a drug manufacturer to enter into and have in effect a National Drug Rebate Agreement NDRA with the Secretary of the Department of Health and Human Services HHS in exchange for state Medicaid coverage of most of the

https://www.govinfo.gov/content/pkg/FR-2020-12-31/pdf/202…
Web 31 d 233 c 2020 nbsp 0183 32 Medicaid beneficiaries The MDRP provides specific requirements for rebate agreements drug pricing submission and confidentiality requirements the formulas for calculating rebate payments drug utilization reviews DUR and requirements for states

Federal Register Medicaid Program Announcement Of Medicaid Drug

Federal Register Medicaid Program Announcement Of Medicaid Drug

Federal Register Medicaid Program Announcement Of Medicaid Drug

Federal Register Medicaid Program Announcement Of Medicaid Drug

TEXAS CHIP

Form 1329 Download Fillable PDF Or Fill Online Kidney Health Care

Form 1329 Download Fillable PDF Or Fill Online Kidney Health Care
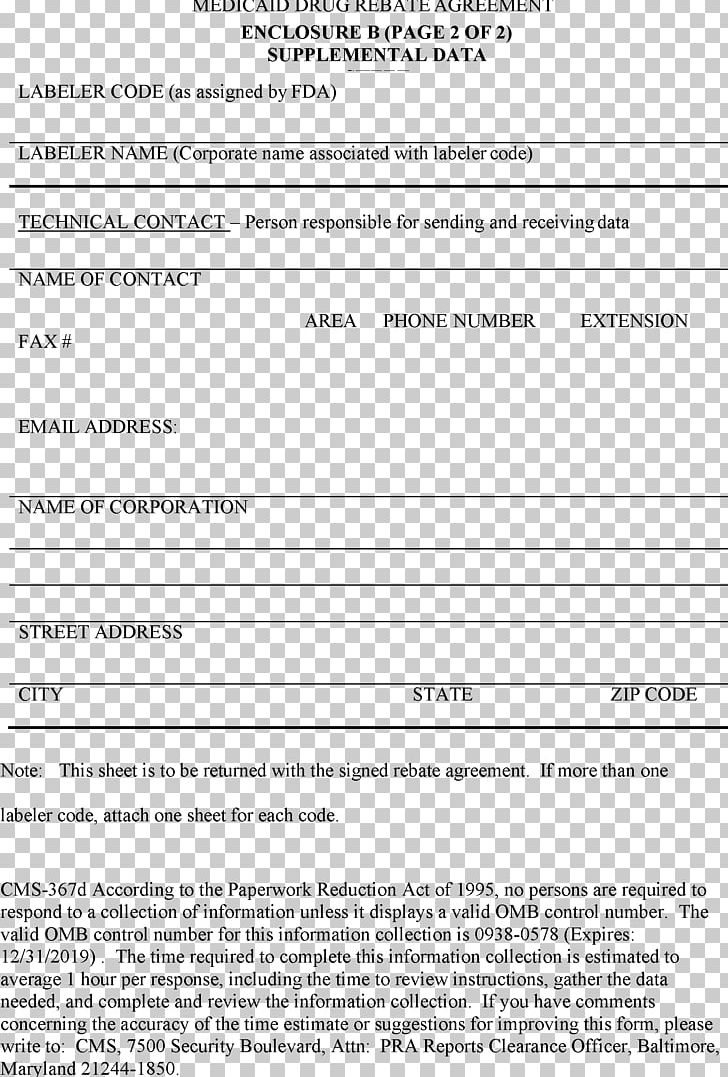
Medicaid Drug Rebate Program Medicare Federal Government Of The United

Federal Register Medicaid Program Announcement Of Medicaid Drug
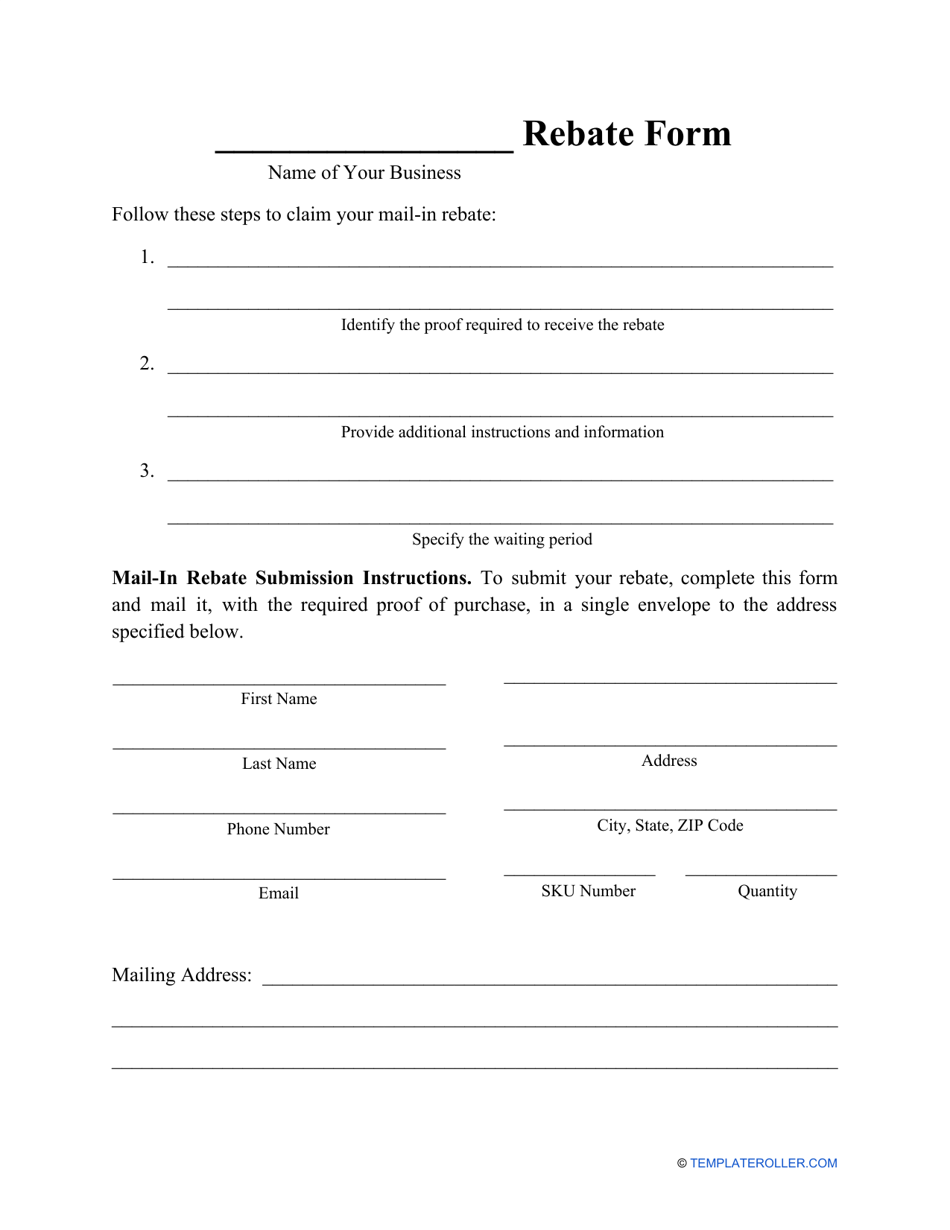
Supplier Rebate Agreement Template
Medicaid Drug Rebate Agreement - Web The Medicaid Drug Rebate Program is a program in the United States that was created by the Omnibus Budget Reconciliation Act of 1990 OBRA 90 The program establishes mandatory rebates that drug manufacturers must pay state Medicaid agencies related to