Orencia Release Date Today the U S Food and Drug Administration approved Orencia abatacept for the prophylaxis prevention of acute graft versus host disease aGVHD a condition that
Abatacept sold under the brand name Orencia is a medication used to treat autoimmune diseases like rheumatoid arthritis by interfering with the immune activity of T cells It is a modified antibody Abatacept is a fusion protein composed of the Fc region of the immunoglobulin IgG1 fused to the extracellular domain of CTLA 4 In order for a T cell to be activated and produce an immune resp ORENCIA is used to reduce signs and symptoms of active Psoriatic Arthritis in people 2 years of age and older In adults ORENCIA may be used alone or with other non
Orencia Release Date

Orencia Release Date
https://clf1.medpagetoday.com/media/images/54xxx/54808.jpg
Panida orencia Shopee Thailand
https://img.ws.mms.shopee.co.th/5dc5a70f87a2c601980fc31216e8e9c5

Buy Orencia Non English Online For 475 Medica Dermal
https://medicadermal.com/wp-content/uploads/2021/12/Orencia_Front.jpg
On December 15 2021 the FDA approved abatacept brand name Orencia for the prophylaxis of acute graft versus host disease in combination with a calcineurin inhibitor Orencia in combination with methotrexate is indicated for the treatment of moderate to severe active rheumatoid arthritis RA in adult patients who responded inadequately to
U S Food and Drug Administration Approves Orencia abatacept in Combination with a Calcineurin Inhibitor and Methotrexate for the Prevention of Acute On Wednesday the U S drug regulator endorsed Orencia for the prevention of acute graft versus host disease GVHD in combination with certain
Download Orencia Release Date
More picture related to Orencia Release Date

ORENCIA FEATHERJET Bristol Myers Squibb Company Trademark Registration
https://uspto.report/TM/77224356/mark.png
Receptor Preparation Tutorial Applications
https://docs.eyesopen.com/applications/_static/logo.svg

Magale Movie 2023 Cast Release Date Story Budget Collection
https://juksun.com/wp-content/uploads/2023/04/Magale-Movie-Poster-scaled.jpg
Orencia Marketing Approval Date 12 15 2021 Approved Labeled Indication prophylaxis of acute graft versus host disease aGVHD in combination with Bristol Myers Squibb Announces Topline Results Showing Treatment with Orencia abatacept Improved Survival in People Hospitalized with COVID 19 The
Bristol Myers Squibb Company NYSE BMY announced today the U S Food and Drug Administration FDA has approved ORENCIA for the treatment of adults with On October 30 2023 the FDA approved Bristol Myers Squibb s Orencia abatacept for the treatment of patients 2 years of age and older with active psoriatic arthritis PsA

Bristol Myers Squibb Launches Orencia ClickJect Drug device Combo For
https://www.drugdeliverybusiness.com/wp-content/uploads/2016/07/clickable-injector.jpg
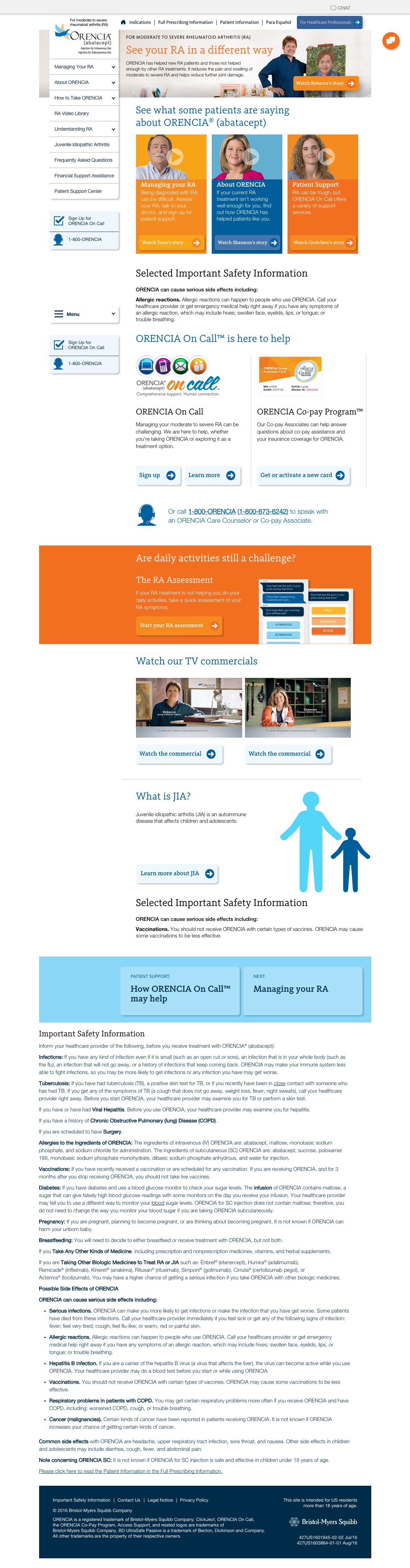
Orencia Patient Website For 2 Indications Once Daily Pharma
https://oncedailypharma.com/wp-content/uploads/2017/04/orencia-home-full-desktop.jpeg

https://www.fda.gov/news-events/press...
Today the U S Food and Drug Administration approved Orencia abatacept for the prophylaxis prevention of acute graft versus host disease aGVHD a condition that

https://en.wikipedia.org/wiki/Abatacept
Abatacept sold under the brand name Orencia is a medication used to treat autoimmune diseases like rheumatoid arthritis by interfering with the immune activity of T cells It is a modified antibody Abatacept is a fusion protein composed of the Fc region of the immunoglobulin IgG1 fused to the extracellular domain of CTLA 4 In order for a T cell to be activated and produce an immune resp

46 Listen Von Europa League Ball 2021 The Official Home Of The Uefa

Bristol Myers Squibb Launches Orencia ClickJect Drug device Combo For

Smalll LBI Health Dept

Comprar Orencia 250 Mg Menor Pre o Entrega R pida

Disney Princess Release Date Movie Disney Princess Photo 32997371

Orencia 101 Uses Side Effects Interactions Plus One Group Of People

Orencia 101 Uses Side Effects Interactions Plus One Group Of People

Blauwal Lehrling Wirtin Jordan1 Og Aufmerksam Betonung Blutung

Rolex Day Date 18038 Champagne Dial Certified Authentic For Sale At

File Hotel Russell On Russell Square London April 2007 jpg
Orencia Release Date - On December 15 2021 the FDA approved abatacept brand name Orencia for the prophylaxis of acute graft versus host disease in combination with a calcineurin inhibitor
