State Of Equilibrium Energy If a reaction is carried out at constant temperature T 0 T 0 then Equation 19 7 1 19 7 1 simplifies to G V P 19 7 2 19 7 2 G V P Under normal conditions the pressure dependence of free energy is not important for solids and liquids because of their small molar volumes
Thermodynamic equilibrium condition or state of a thermodynamic system the properties of which do not change with time and that can be changed to another condition only at the expense of effects on other systems For a thermodynamic equilibrium system with given energy the entropy is greater The Road to Equilibrium is Down the Gibbs Energy Hill This means of course that if the total Gibbs energy G of a mixture of reactants and products goes through a minimum value as the composition changes then all net change will cease the reaction system will be in a state of chemical equilibrium
State Of Equilibrium Energy

State Of Equilibrium Energy
https://3.bp.blogspot.com/-QmrypClubyA/WpHOSzjKQhI/AAAAAAAAAJs/T7BonlkY6sAgy07NA3YQWavhs2EoHBJEQCLcBGAs/s1600/Seesaw%2Bat%2BStatic%2BEquilibrium.png
Why Equilibrium Constants Are Unitless The Journal Of Physical
https://pubs.acs.org/cms/10.1021/acs.jpclett.2c00314/asset/images/acs.jpclett.2c00314.social.jpeg_v03

Gibbs Free Energy Definition Equation Unit And Example
https://www.chemistrylearner.com/wp-content/uploads/2022/01/Gibbs-Free-Energy-Graph.jpg
Equilibrium in physics the condition of a system when neither its state of motion nor its internal energy state tends to change with time A simple mechanical body is said to be in equilibrium if it experiences neither linear acceleration nor angular acceleration unless it is disturbed by an Gibbs Energy is a state function defined as G H TS G H T S The practical utility of the Gibbs function is that G G for any process is negative if it leads to an increase in the entropy of the world Thus spontaneous change at a given temperature and pressure can only occur when it would lead to a decrease in G G
The standard change in free energy G for a reaction is related to its equilibrium constant K by the equation G RTlnK When G 0 K 1 and the reaction is product favored at equilibrium When G 0 K 1 and the reaction is reactant favored at equilibrium Thermodynamics Equilibrium Heat Energy Britannica Contents Home Science Physics Thermodynamic equilibrium A particularly important concept is thermodynamic equilibrium in which there is no tendency for the state of a system to change spontaneously
Download State Of Equilibrium Energy
More picture related to State Of Equilibrium Energy

Equilibrium Price EQ Price Index Live Chart And USD Converter Binance
https://s2.coinmarketcap.com/static/img/coins/64x64/6780.png

Conditions Of Equilibrium Concept Mechanical Engineering JoVe
https://cloudfront.jove.com/files/media/science-education/science-education-thumbs/14269.jpg

14 1 The Equilibrium State YouTube
https://i.ytimg.com/vi/vA41RhGc28I/maxresdefault.jpg
Equilibrium Thermodynamics is the systematic study of transformations of matter and energy in systems in terms of a concept called thermodynamic equilibrium The word equilibrium implies a state of balance Equilibrium thermodynamics in origins derives from analysis of the Carnot cycle V Parameswaran Nair City College of New York The second law of thermodynamics implies that entropy does not decrease in any natural process The final equilibrium state will thus be the state of maximum possible entropy After attaining this maximum possible value the entropy will remain constant
Thus the equilibrium point is given by the condition that the force associated with the potential is zero x 0 in the case of the potential energy from a spring The equilibrium is a stable equilibrium because the force associated with the potential energy function F x kx for the spring points towards the equilibrium point State the types of equilibrium Describe stable and unstable equilibriums Describe neutral equilibrium It is one thing to have a system in equilibrium it is quite another for it to be stable The toy doll perched on the man s hand in Figure 9 3 1 9 3 1 for example is not in stable equilibrium
Chemical Equilibrium
http://yarathermowarriors.weebly.com/uploads/3/8/1/4/38142221/9620902_orig.png
:max_bytes(150000):strip_icc()/Equilibrium_Version1_4197021-488ad218b7a9456e96ea400c6588359c.png)
Equilibrium Price Definition Types Example And How To Calculate
https://www.investopedia.com/thmb/vDbq4ragGhfZ9dv-Q6xWSIB-dvQ=/1500x0/filters:no_upscale():max_bytes(150000):strip_icc()/Equilibrium_Version1_4197021-488ad218b7a9456e96ea400c6588359c.png
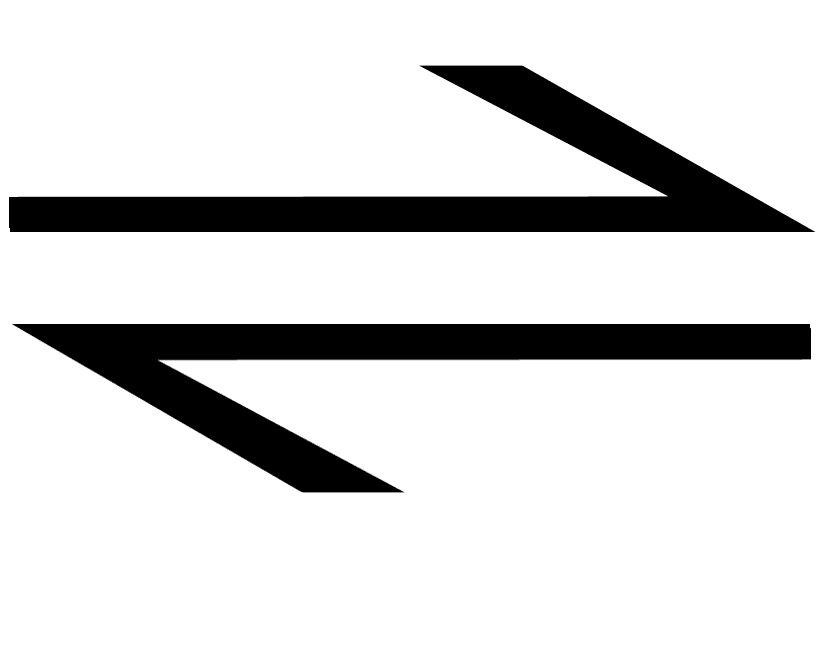
https://chem.libretexts.org/Bookshelves/General...
If a reaction is carried out at constant temperature T 0 T 0 then Equation 19 7 1 19 7 1 simplifies to G V P 19 7 2 19 7 2 G V P Under normal conditions the pressure dependence of free energy is not important for solids and liquids because of their small molar volumes

https://www.britannica.com/science/thermodynamic-equilibrium
Thermodynamic equilibrium condition or state of a thermodynamic system the properties of which do not change with time and that can be changed to another condition only at the expense of effects on other systems For a thermodynamic equilibrium system with given energy the entropy is greater

Difference Between Equilibrium And Steady State Compare The

Chemical Equilibrium

Equilibrium 03 Tikal

What Is Market Equilibrium Definition Example Parsadi
Physics Equilibrium
Working For Equilibrium Stumbling After Francis

Working For Equilibrium Stumbling After Francis

Equilibrium Official Website Pictures

Forces In Equilibrium

W02M04 Dynamic Equilibrium Equation By Energy Method YouTube
State Of Equilibrium Energy - In other words in the equilibrium state the energy Eq 6 is an integral 12 E 1 1 F 1 d 1 1 1 d 1 It could be proposed that the external field 1 is given while the term V 1 characterizes the thermodynamic properties of the electronic system
