What Is Activation Energy Class 12 Chemistry Activation energy is the least possible energy required to start a chemical reaction The activation energy Ea of a reaction is measured in joules J
Threshold Energy It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming the products Activation Energy It Activation Energy is the energy which must be provided to potential reactants Some chemical reactions start as soon as the reactants come into contact
What Is Activation Energy Class 12 Chemistry

What Is Activation Energy Class 12 Chemistry
https://sciencenotes.org/wp-content/uploads/2021/03/Activation-Energy-768x512.jpg

Iconpasob blogg se How Much Does Catalyst Need Of Activation Energy
https://lightcat-files.s3.amazonaws.com/problem_images/6b11776b92ca886d-1569863624014.jpg

Activation Energy Examples
https://cdn1.byjus.com/wp-content/uploads/2023/02/Activation-Energy-2.png
According to the concept of activation energy the reactants first absorb energy equal to activation energy and thereby form activated complex At this state the molecules have energy at least equal to the threshold energy The base energy prerequisite that must be met for a chemical reaction to happen is known as the activation energy It can be represented as E a Note We have to remember that the
Activation energy refers to the minimum amount of energy needed that can bring about the activation of atoms or molecules to a condition where they can perform a chemical transformation or physical transport Activation energy is the minimum energy required by the molecules to react and produce the expected products The S I unit of activation energy is Joules J and in moles it is Kj mol
Download What Is Activation Energy Class 12 Chemistry
More picture related to What Is Activation Energy Class 12 Chemistry
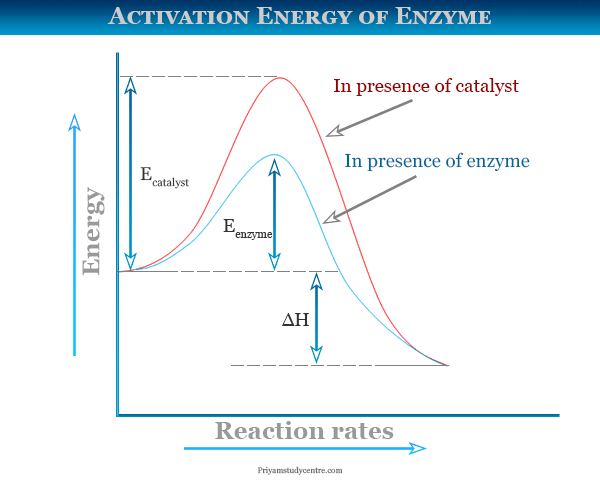
Enzyme Activation Energy Graph
https://www.priyamstudycentre.com/wp-content/uploads/2020/09/activation-energy-of-enzyme.png

GCSE Chemistry 1 9 What Is Activation Energy YouTube
https://i.ytimg.com/vi/VFjrOzmPELg/maxresdefault.jpg
Activation Energy Liberal Dictionary
https://www.tekportal.net/wp-content/uploads/2019/01/activation-energy-6313.jpg
Full syllabus notes lecture and questions for Energy of Activation Chemical Kinetics Class 12 Chemistry JEE JEE Plus excerises question with solution to help you revise complete What is activation energy in chemical kinetics Ans Activation energy is the minimum amount of energy required for a chemical reaction to occur It is the energy barrier that must be
The energy required to form the intermediate called activated complex is known as activation energy Activation energy Threshold energy Average energy of the reactants Was this In chemistry and physics activation energy is the minimum amount of energy needed to start a chemical reaction Reactants often get activation energy from heat but
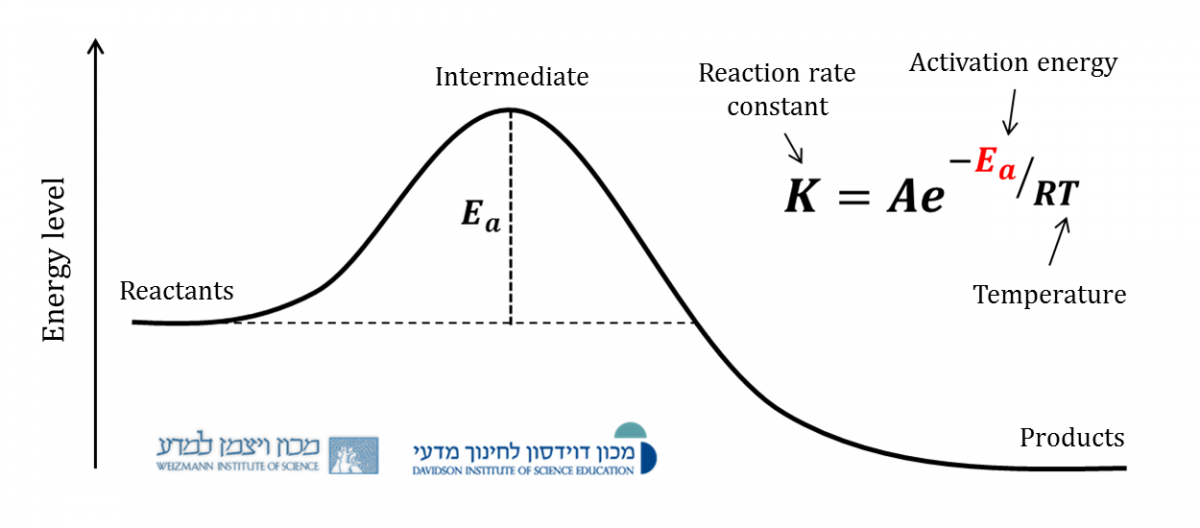
Activation Energy Arrhenius Equation
http://cimg1.ck12.org/datastreams/f-d:df0a2687d885c997ec852a60b09181c51b0a234ada9136e0288d4e8c%2BIMAGE%2BIMAGE.1
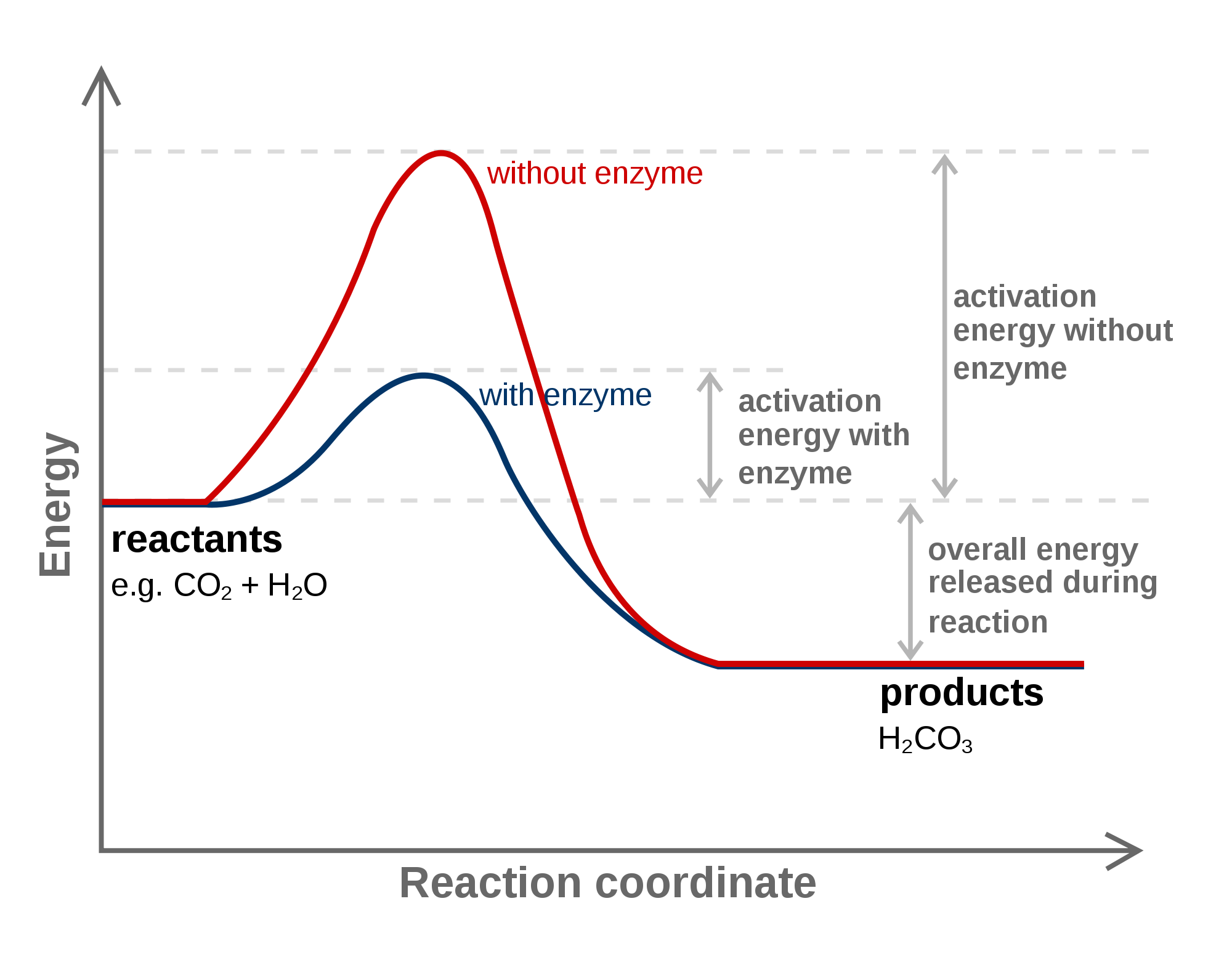
Enzymes Activation Energy
https://useruploads.socratic.org/olM9lPWPSziwQey3xmTP_2000px-Carbonic_anhydrase_reaction_in_tissue.svg.png

https://byjus.com › activation-energy-form…
Activation energy is the least possible energy required to start a chemical reaction The activation energy Ea of a reaction is measured in joules J

https://www.sarthaks.com › define-threshold...
Threshold Energy It is the minimum amount of energy which the reactant molecules must possess for the effective collision in forming the products Activation Energy It
Activation Energy The Secret To Getting Started And Getting Finished
Activation Energy Arrhenius Equation

6 2 2 Define The Term Activation Energy E A YouTube

Activation Energy Vector Illustration Example Diagram Physics And
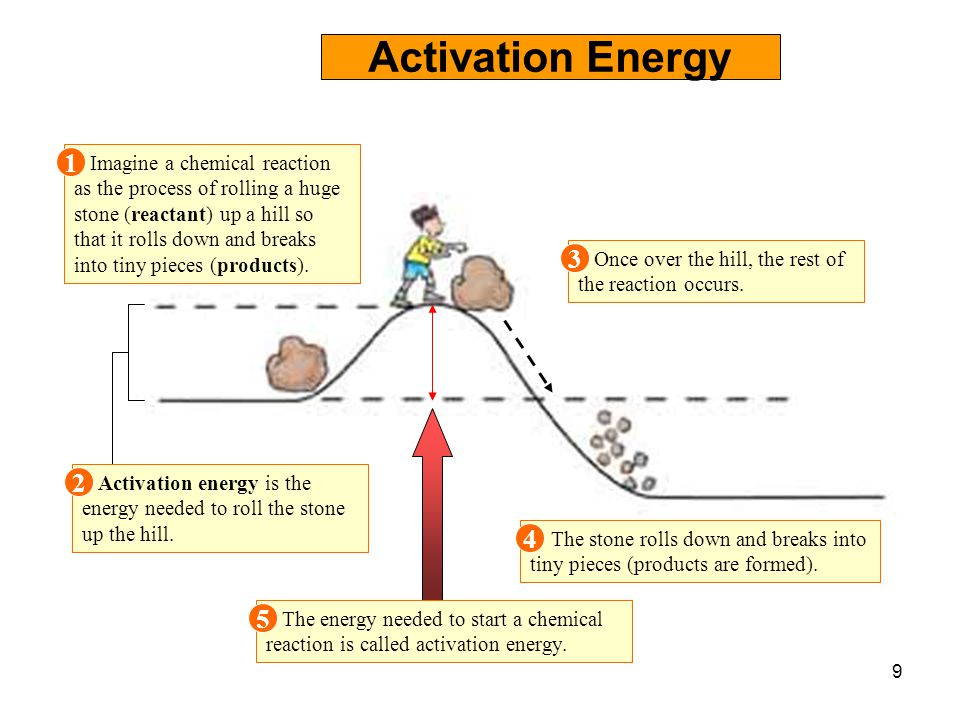
Activation Energy The Secret To Getting Started And Getting Finished

Activation Energy Chemistry Education Learning Science Chemistry

Activation Energy Chemistry Education Learning Science Chemistry
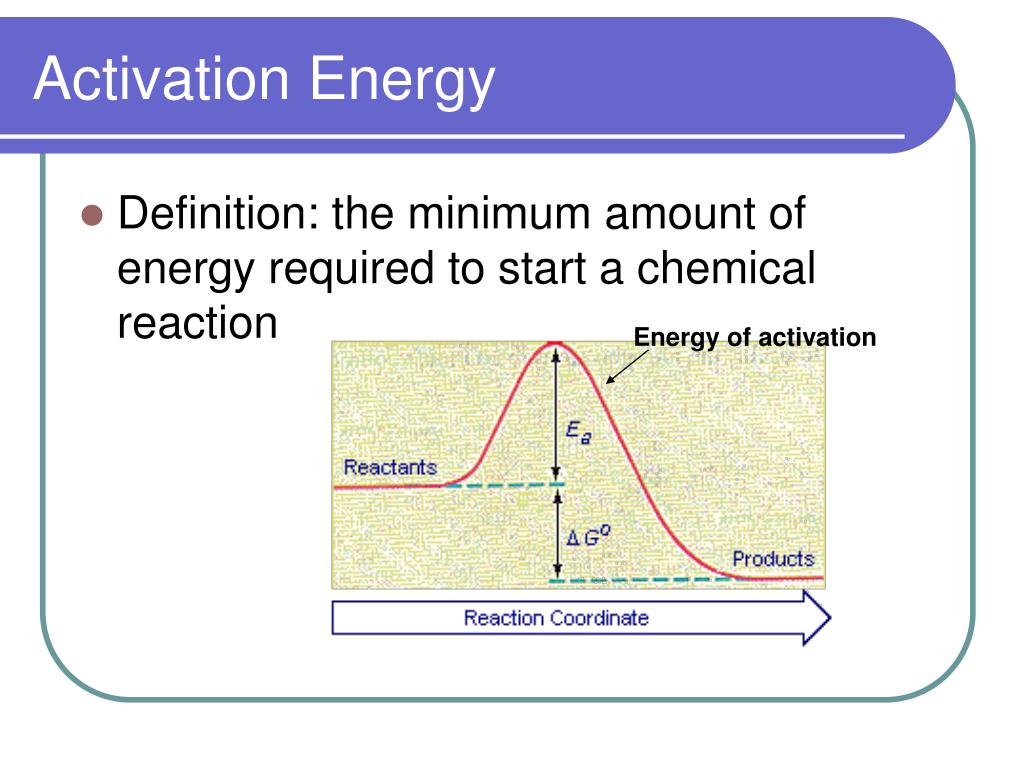
Activation Energy For Diffusion Controlled Reaction
1 15 Energy And Biochemical Reactions Biology LibreTexts

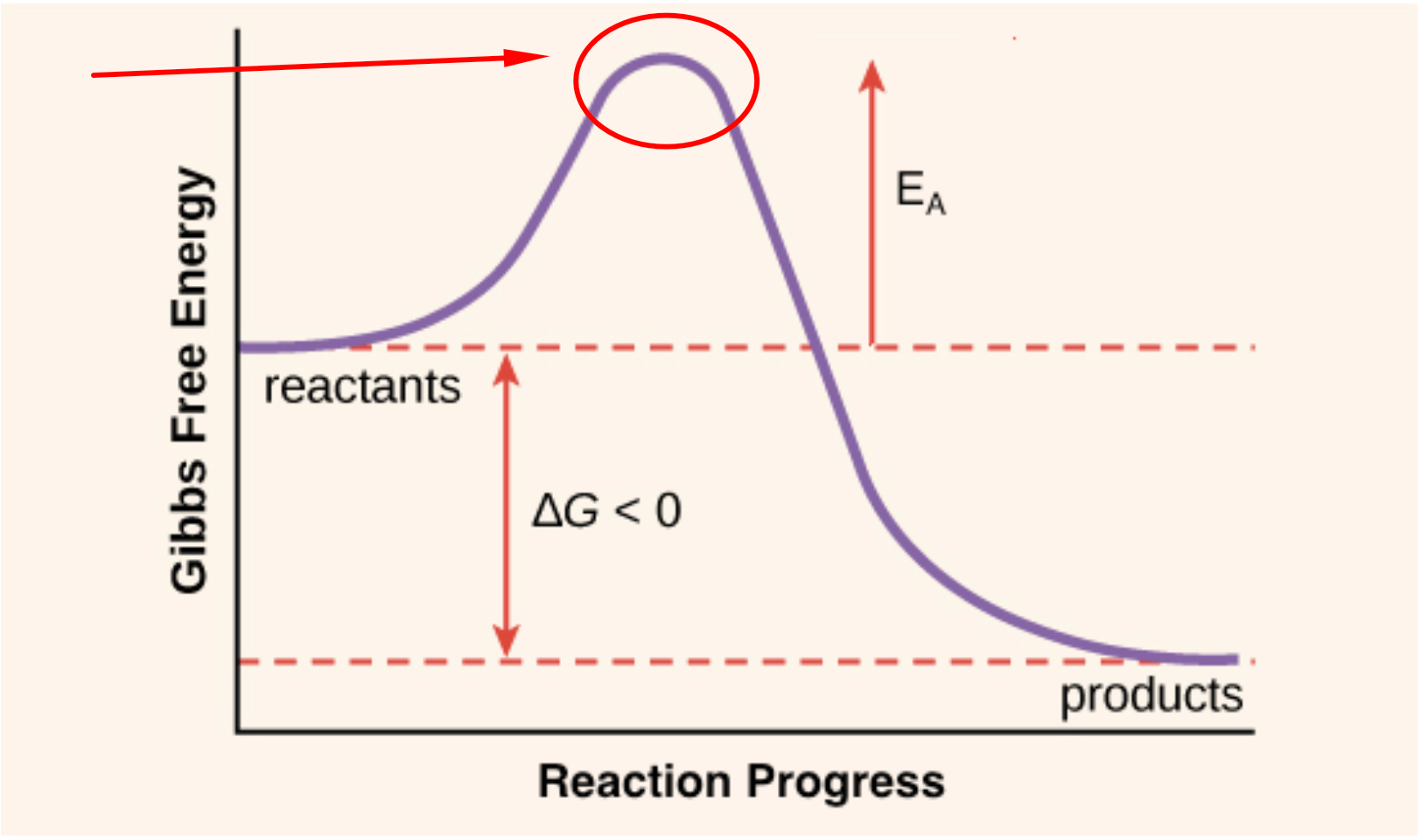
Kinetics Why Is Activation Energy Drawn In A Potential Energy Diagram
What Is Activation Energy Class 12 Chemistry - In chemistry activation energy is the minimum amount of energy required for a chemical reaction The activation energy can be thought of as the magnitude of a potential barrier that the