What Is Internal Energy Internal energy is a state function of a system and is an extensive quantity One can have a corresponding intensive thermodynamic property called specific internal energy commonly symbolized by the lowercase letter u which is internal energy per mass of the substance in question
In chemistry and physics internal energy U is defined as the total energy of a closed system Internal energy is the sum of potential energy of the system and the system s kinetic energy Internal energy in thermodynamics the property or state function that defines the energy of a substance in the absence of effects due to capillarity and external electric magnetic and other fields Like any other state function the value of the energy depends upon the state of the substance
What Is Internal Energy
What Is Internal Energy
https://eduinput.com/wp-content/uploads/2022/08/feature-image-of-internal-energy.jpg

PPT Thermodynamics PowerPoint Presentation Free Download ID 1607569
https://image1.slideserve.com/1607569/what-is-internal-energy-l.jpg
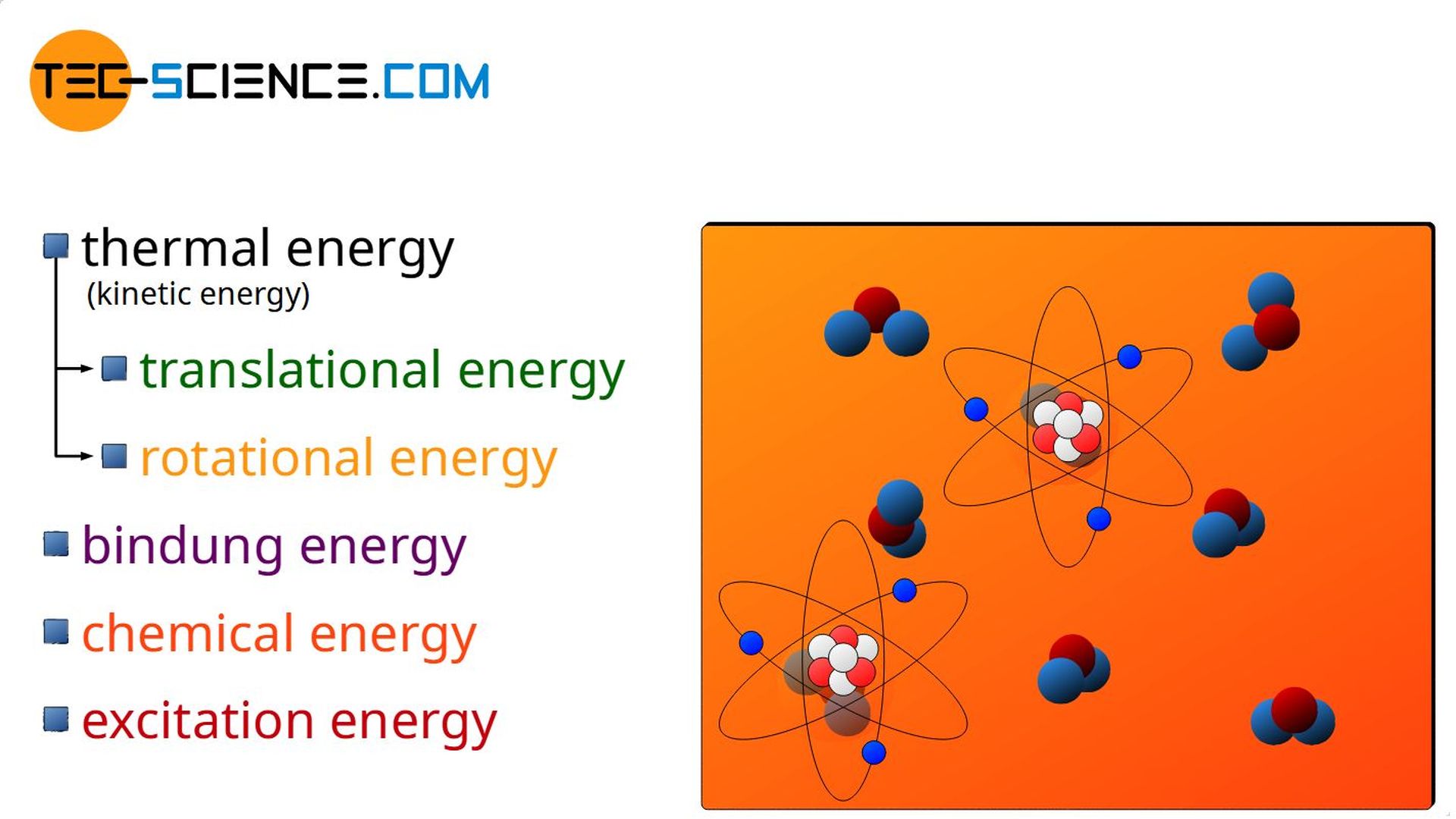
Internal Energy First Law Of Thermodynamics Tec science
https://www.tec-science.com/wp-content/uploads/2021/05/en-thermodynamics-thermodynamic-processes-internal-energy-excitation-energy.jpg
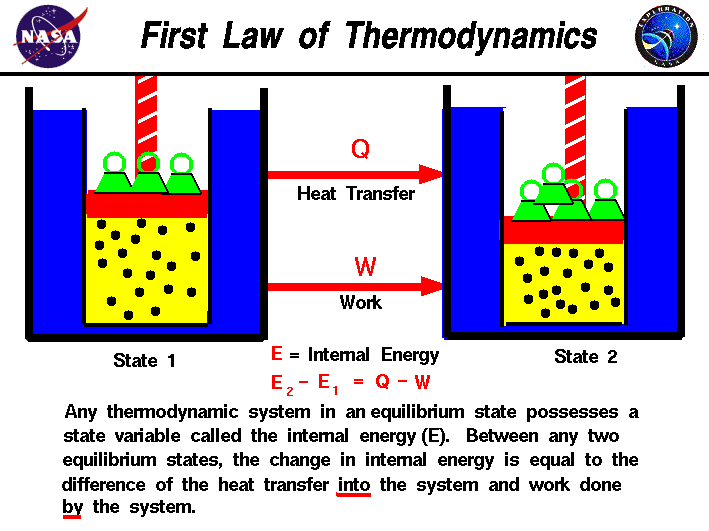
A reaction or process in which heat is transferred to a system from its surroundings is endothermic The first law of thermodynamics states that the energy of the universe is constant The change in the internal energy of a system is the sum of the heat transferred and the work done The internal energy of a system is identified with the random disordered motion of molecules the total internal energy in a system includes potential and kinetic energy This is contrast to external energy which is a function of the sample with respect to the outside environment e g kinetic energy if the sample is moving or potential
The sum of all the different kinds of energy which the molecules of a substance can possess is called the internal energy and given the symbol U The symbol E also widely used In a gas we can regard the internal energy as the sum of the electronic translational rotational and vibrational energies Changes in a material s temperature or state of matter are caused by changes to the internal energy The energy required by different materials depends on their
Download What Is Internal Energy
More picture related to What Is Internal Energy
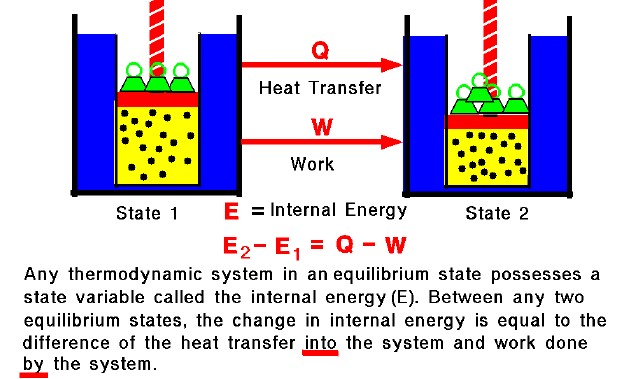
First Law Internal Energy Glenn Research Center NASA
https://www1.grc.nasa.gov/wp-content/uploads/thermo1-3.jpg

Thermodynamics Internal Energy YouTube
https://i.ytimg.com/vi/8E2aImEpz-o/maxresdefault.jpg
What Is Internal Energy Definition And Example
https://eduinput.com/wp-content/uploads/2022/08/image-of-internal-energy-01.png
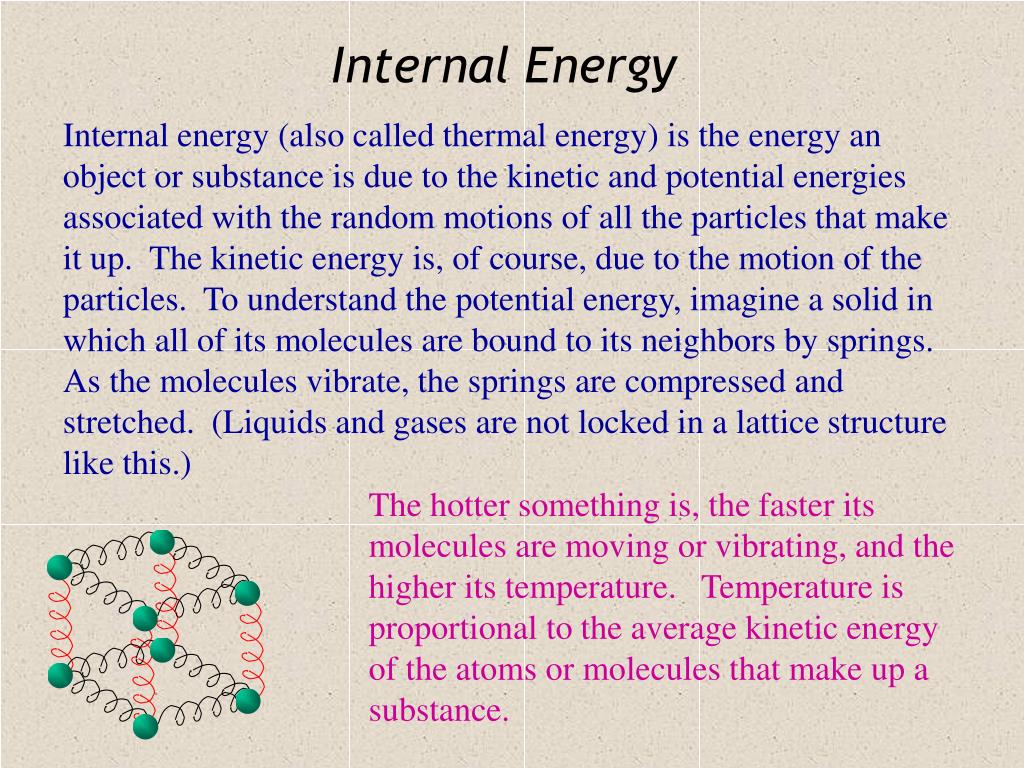
Internal energy is defined as the energy associated with the random disordered motion of molecules It is separated in scale from the macroscopic ordered energy associated with moving objects it refers to the invisible microscopic energy on The internal energy of an object is intrinsically related to its temperature When a container containing gas molecules is heated up the molecules begin to move around faster increasing their kinetic energy If the object is a solid where the molecules are tightly packed when heated the molecules begin to vibrate more
[desc-10] [desc-11]
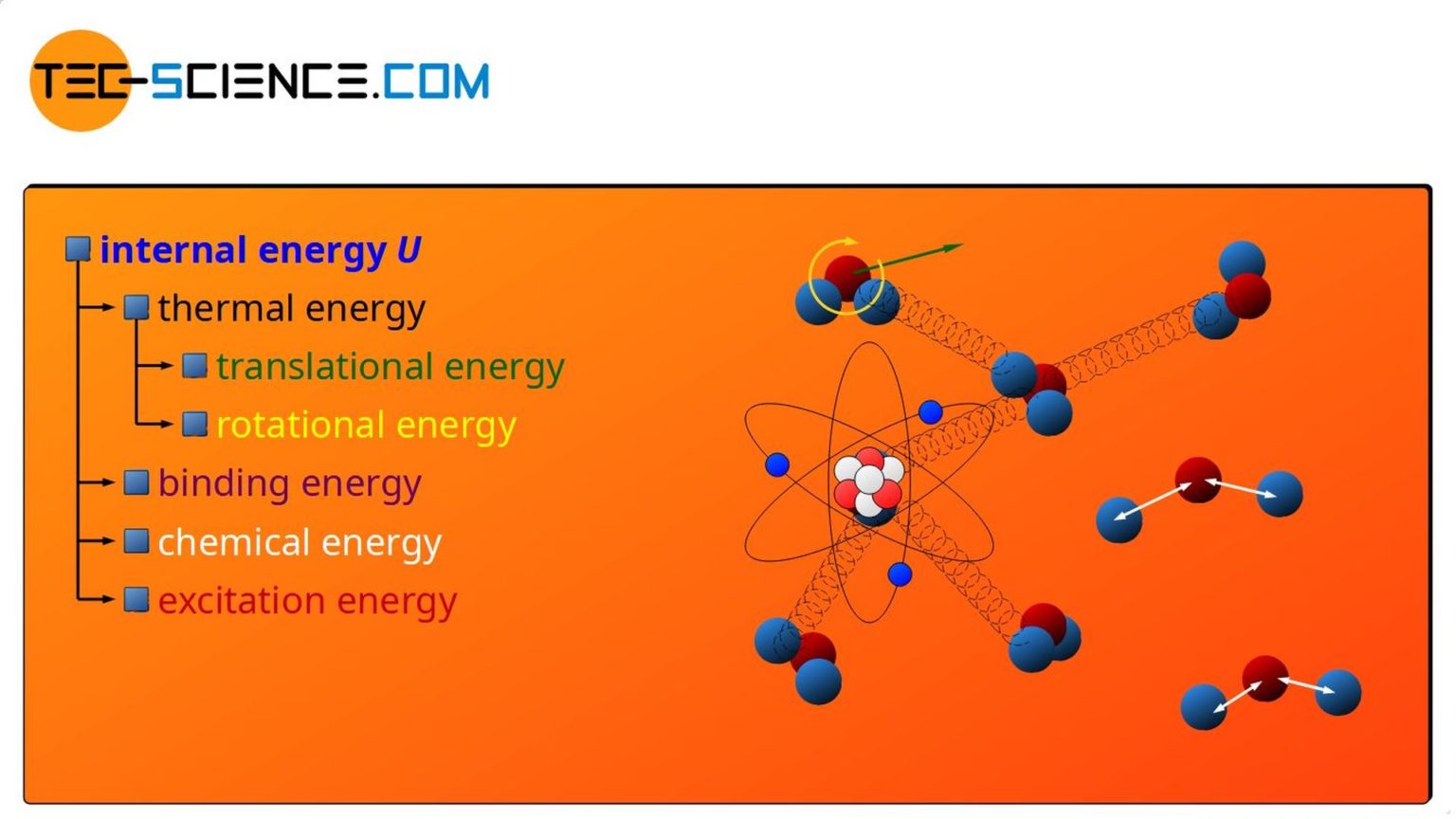
Internal Energy First Law Of Thermodynamics Tec science
https://www.tec-science.com/wp-content/uploads/2021/05/en-thermodynamics-thermodynamic-processes-internal-energy-types-1536x864.jpg
First Law Of Thermodynamics
https://www.grc.nasa.gov/www/k-12/rocket/Images/thermo1.gif
https://byjus.com/chemistry/internal-energy
Internal energy is a state function of a system and is an extensive quantity One can have a corresponding intensive thermodynamic property called specific internal energy commonly symbolized by the lowercase letter u which is internal energy per mass of the substance in question

https://www.thoughtco.com/definition-of-internal-energy-605254
In chemistry and physics internal energy U is defined as the total energy of a closed system Internal energy is the sum of potential energy of the system and the system s kinetic energy
PPT Thermodynamics PowerPoint Presentation Free Download ID 6936340

Internal Energy First Law Of Thermodynamics Tec science

Internal Energy YouTube

Thermodynamics 1 C2 L3 Internal Energy YouTube

Internal Energy GCSE Physics Revision
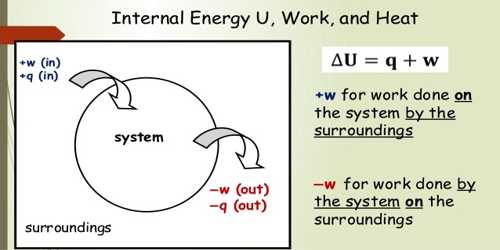
Internal Energy Of A System QS Study

Internal Energy Of A System QS Study
PPT Chapter 20 PowerPoint Presentation Free Download ID 5988619

What Is Internal Energy YouTube
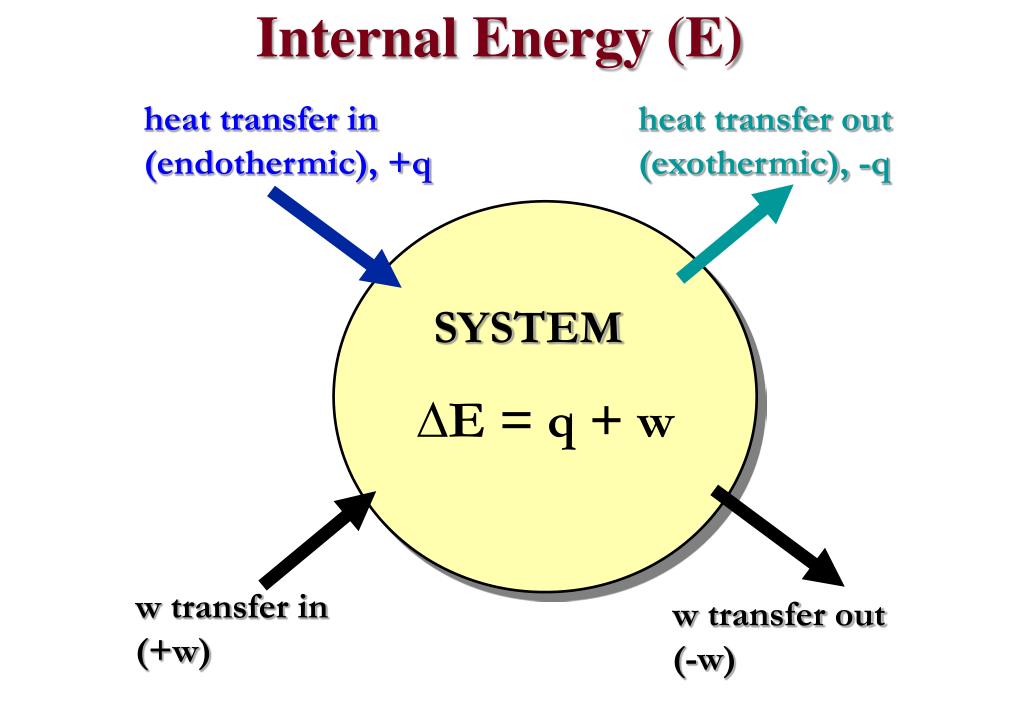
PPT Energy Chemistry PowerPoint Presentation Free Download ID
What Is Internal Energy - A reaction or process in which heat is transferred to a system from its surroundings is endothermic The first law of thermodynamics states that the energy of the universe is constant The change in the internal energy of a system is the sum of the heat transferred and the work done