Ich Gcp Guidelines 2023 This document addresses the good clinical practice an international ethical and scientific quality standard for designing conducting recording and reporting trials that involve the participation of human subjects
The ICH E6 R3 draft Guideline on Good Clinical Practice GCP has reached Step 2 of the ICH process on 19 May 2023 The E6 R3 draft Guideline and guidance for stakeholder public consultation comment collection are available for download on the E6 R3 page ICH E6 Good Clinical Practice GCP Guideline is widely used by clinical trial researchers beyond the membership and regional representation of ICH itself and has a significant impact on trial participants and patients
Ich Gcp Guidelines 2023

Ich Gcp Guidelines 2023
https://i.ytimg.com/vi/TCW55krWzb4/maxresdefault.jpg
The Importance Of ICH GCP CCRPS
http://static1.squarespace.com/static/56a042fb25981d9326c9bbdb/t/6091584d9efd40482ae228c5/1620138074539/ich+gcp?format=1500w

ICH GCP Certificate Nhvm NIDA Clinical Trials Network Certificate
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/a87f4371c3e56c3e0e3972c947ae330e/thumb_1200_1553.png
This draft guidance encourages innovation focuses on quality and establishes proportionate and risk based approaches for conducting clinical trials while minimizing unnecessary complexities The ICH E6 R3 draft Guideline on Good Clinical Practice reached Step 2b of the ICH Process in May 2023 and subsequently entered the consultation period A Step 2 Informational Presentation has been developed by the Expert Working Group and is now available for download on the Efficacy Guidelines page About ICH
Good Clinical Practice GCP Step 1 Final Concept Paper ICH E6 R3 Guideline for Good Clinical Practice Annex 2 30 March 2023 Endorsed by the Management Committee on 28 April 2023 Type of Harmonisation Action Proposed This ICH GCP Guidance Integrated Addendum provides a unified standard for the European Union Japan the United States Canada and Switzerland to facilitate the mutual acceptance of data
Download Ich Gcp Guidelines 2023
More picture related to Ich Gcp Guidelines 2023
Introduction To ICH GCP
https://assets.api.gamma.app/d7uc3xhun79v8gv/screenshots/0gzq6r35xrhxdla/kx4evwxpa6xmr4f/slide/AUkjE3JJiZsb2IkQs1UumxbweaU

2023 CMS Pain Management Codes Www drbillingservice
https://www.drbillingservice.com/blog/wp-content/uploads/2023/04/15.jpg
GCP VScode
https://tistory1.daumcdn.net/tistory/5980396/attach/a04e3b50c3dd4c3ba4f07af6c2add421
Training components that should be included to make global GCP training useful as the EWG is planning to develop training materials for ICH E6 R3 The highly anticipated draft of the International Council for Harmonisation ICH E6 R3 Guideline has now been published In the knowledge that some content may be revised here is a summary of the changes Key changes A revised layout
[desc-10] [desc-11]
![]()
AASP 2023
https://files.sciconf.cn/meeting/2023/17010/image/20230131/2023013114260251437982610.gif
Making Good Clinical Practice More Understandable Advarra
https://info.advarra.com/rs/291-FFI-055/images/GCP-eb-featured.png

https://www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice
This document addresses the good clinical practice an international ethical and scientific quality standard for designing conducting recording and reporting trials that involve the participation of human subjects

https://ich.org/news/ich-e6r3-draft-guideline-reaches-step-2-ich-process
The ICH E6 R3 draft Guideline on Good Clinical Practice GCP has reached Step 2 of the ICH process on 19 May 2023 The E6 R3 draft Guideline and guidance for stakeholder public consultation comment collection are available for download on the E6 R3 page

How To Reduce IT Infrastructure Cost With GCP Dicoding Indonesia
AASP 2023

ICH GCP 2023 ICH GCP 2023
SURE 2023 Clustering K means

Vol 1 No 5 2023 June 2023 International Journal Of Scientific

DML 2023 Canada

DML 2023 Canada

Tasmanian Palliative Care Awards 2023 Palliative Care Tasmania
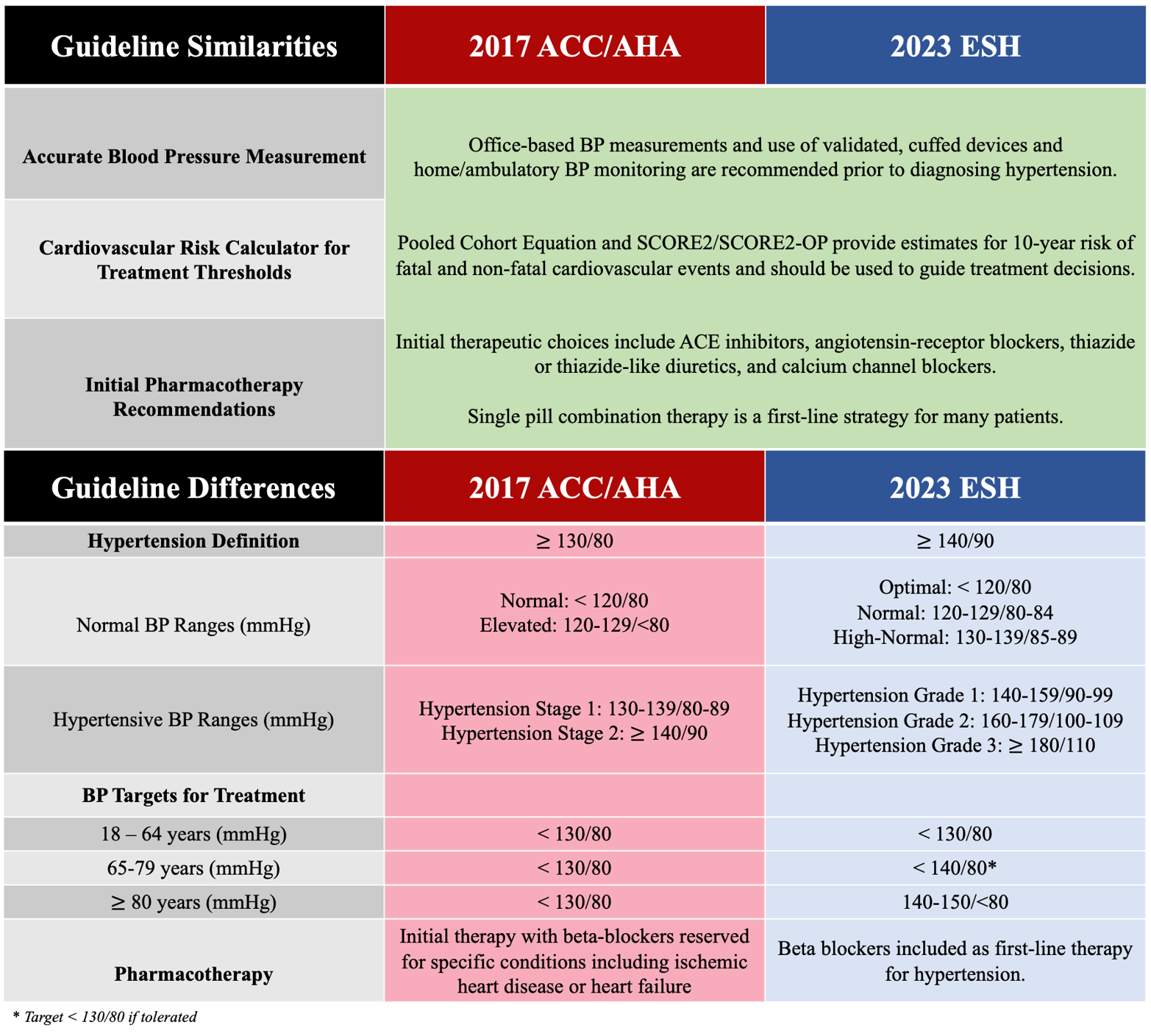
2023 ESH Hypertension Guideline Update Bringing Us Closer Together

Policy And Guidelines Text
Ich Gcp Guidelines 2023 - [desc-14]


