Medicaid Drug Rebate Program Agreement Web 11 ao 251 t 2022 nbsp 0183 32 The program requires a drug manufacturer to enter into and have in effect a National Drug Rebate Agreement NDRA with the Secretary of the Department of
The Medicaid Drug Rebate Program is a program in the United States that was created by the Omnibus Budget Reconciliation Act of 1990 OBRA 90 The program establishes mandatory rebates that drug manufacturers must pay state Medicaid agencies related to the dispensing of outpatient prescription drugs covered by Medicaid To participate in the program drug manufacturers must have a National Drug Rebate Agreement wit Web 12 sept 2023 nbsp 0183 32 Medicaid Drug Rebate Program Consumer Price Index Urban Value Dispute Resolution File Formats and Data Definitions Interest Calculation for Late
Medicaid Drug Rebate Program Agreement

Medicaid Drug Rebate Program Agreement
https://s3.amazonaws.com/images.federalregister.gov/EN23MR18.002/original.png?1521650112

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN09NO16.006/original.png?1481698007

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN23MR18.008/original.png?1521650113
Web 8 janv 2021 nbsp 0183 32 January 8 2021 Covington Alert On December 31 2020 the Centers for Medicare amp Medicaid Services CMS published in the Federal Register a final rule Web 10 f 233 vr 2023 nbsp 0183 32 The statements included on this web page are intended to provide information on Medicaid Drug Rebate Program Releases and do not in any way revise
Web 1 EXECUTIVE SUMMARY This white paper intends to offer the pharmaceutical industry an overview of the Medicaid Drug Rebate Program MDRP also known as the Omnibus Web 14 juil 2022 nbsp 0183 32 CMS implemented a new Medicaid Drug Programs MDP system in 2021 We have posted the MDP file formats as well as state and manufacturer email
Download Medicaid Drug Rebate Program Agreement
More picture related to Medicaid Drug Rebate Program Agreement

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN09NO16.010/original.png?1478266589

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN23MR18.009/original.png?1521650113

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN23MR18.000/original.png?1521650111
Web Under the Medicaid Drug Rebate Program a drug manufacturer must enter into a Medicaid national drug rebate agreement with the Secretary of the U S Department of Web 6 juil 2016 nbsp 0183 32 Congress created the Medicaid Drug Rebate Program to reduce Medicaid s expenditures on prescription drugs The program has successfully provided Medicaid with the lowest net prices for
Web 28 mai 2021 nbsp 0183 32 rule entitled Medicaid Program Establishing Minimum Standards in Medicaid State Drug Utilization Review DUR and Supporting Value Based Purchasing Web 29 mars 2022 nbsp 0183 32 On March 23 2022 the Centers for Medicare and Medicaid Services CMS issued long awaited guidance regarding how drug manufacturers are to report

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN23MR18.006/original.png?1521650112

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN09NO16.002/original.png?1481697994

https://www.medicaid.gov/.../medicaid-drug-rebate-program/index.html
Web 11 ao 251 t 2022 nbsp 0183 32 The program requires a drug manufacturer to enter into and have in effect a National Drug Rebate Agreement NDRA with the Secretary of the Department of

https://en.wikipedia.org/wiki/Medicaid_Drug_Rebate_Program
The Medicaid Drug Rebate Program is a program in the United States that was created by the Omnibus Budget Reconciliation Act of 1990 OBRA 90 The program establishes mandatory rebates that drug manufacturers must pay state Medicaid agencies related to the dispensing of outpatient prescription drugs covered by Medicaid To participate in the program drug manufacturers must have a National Drug Rebate Agreement wit

Federal Register Medicaid Program Announcement Of Medicaid Drug

Federal Register Medicaid Program Announcement Of Medicaid Drug

Federal Register Medicaid Program Announcement Of Medicaid Drug

TEXAS CHIP

Federal Register Medicaid Program Announcement Of Medicaid Drug
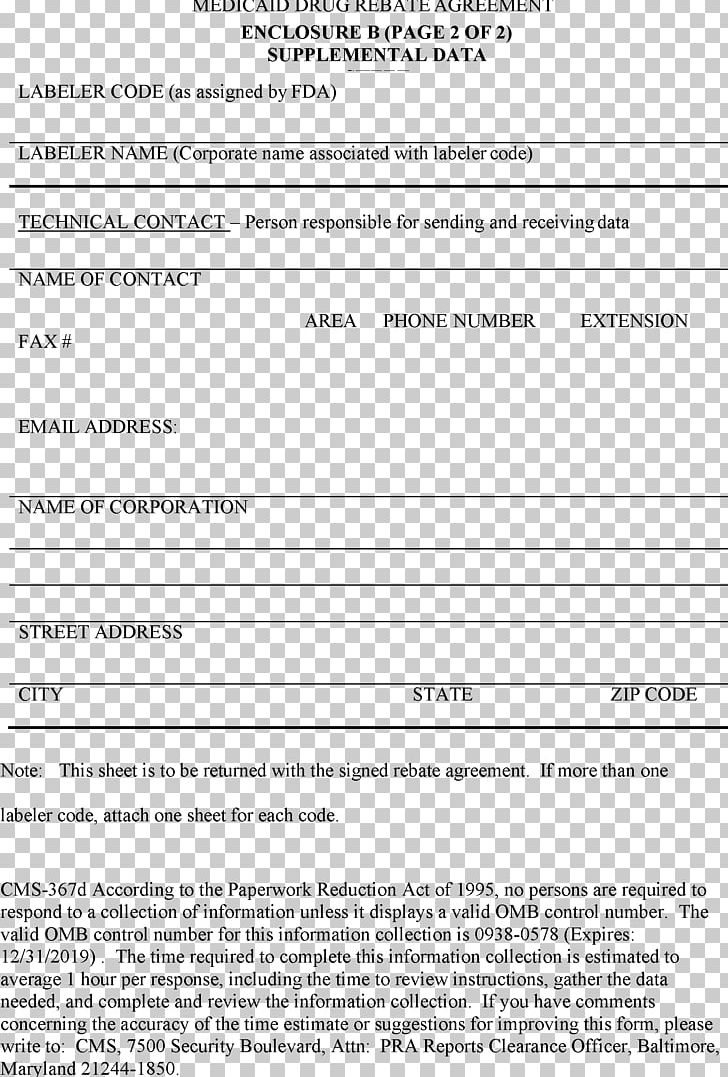
Medicaid Drug Rebate Program Medicare Federal Government Of The United

Medicaid Drug Rebate Program Medicare Federal Government Of The United

Federal Register Medicaid Program Announcement Of Medicaid Drug

Form 1329 Download Fillable PDF Or Fill Online Kidney Health Care
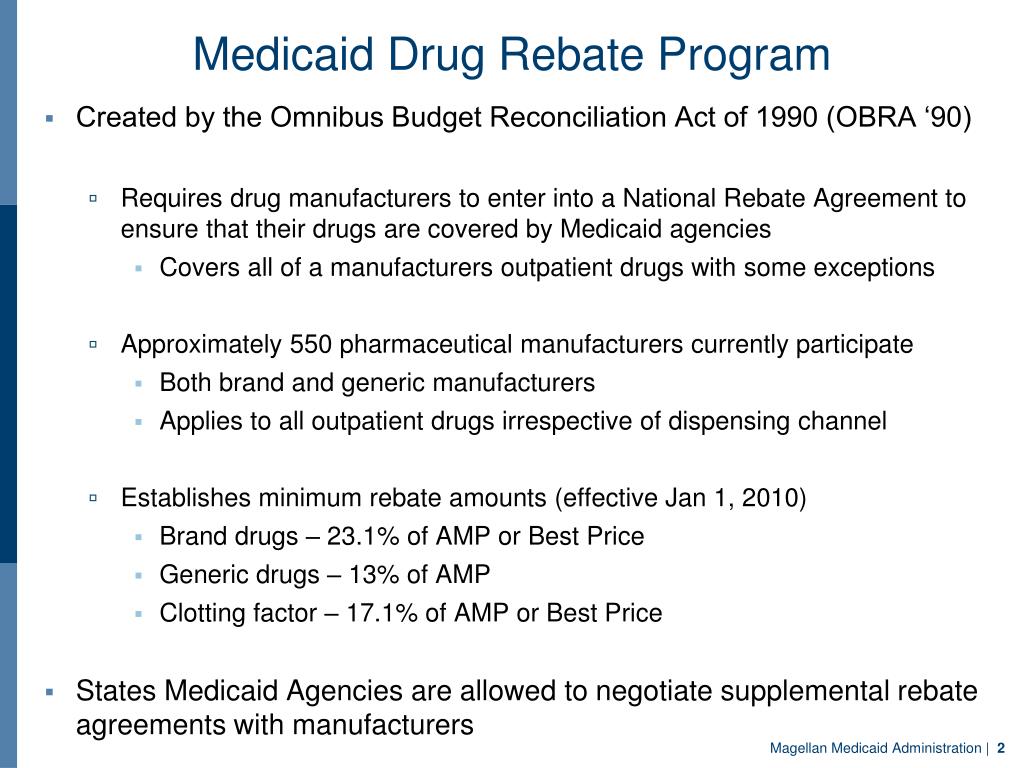
PPT Medicaid Drug Rebate Program PowerPoint Presentation Free
Medicaid Drug Rebate Program Agreement - Web The law establishes Medicare Part B prescription drug inflation rebates for single source drugs and biologicals with prices increasing faster than the rate of inflation and provides