Medicaid Rebate Agreement Web 15 juin 2023 nbsp 0183 32 The Medicaid rebate statute 2 requires manufacturers of covered outpatient drugs to enter into a rebate agreement with CMS as a condition to federal funding for
Web 11 ao 251 t 2022 nbsp 0183 32 The program requires a drug manufacturer to enter into and have in effect a National Drug Rebate Agreement NDRA with the Secretary of the Department of Web 5 sept 2023 nbsp 0183 32 Medicaid Drug Rebate Program Consumer Price Index Urban Value Dispute Resolution File Formats and Data Definitions Interest Calculation for Late
Medicaid Rebate Agreement

Medicaid Rebate Agreement
https://s3.amazonaws.com/images.federalregister.gov/EN23MR18.002/original.png?1521650112

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN23MR18.000/original.png?1521650111

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN09NO16.008/original.png?1481698012
Web 23 mars 2018 nbsp 0183 32 This final notice announces changes to the Medicaid National Drug Rebate Agreement NDRA or Agreement for use by the Secretary of the Department of Health and Human Services HHS and Web 8 janv 2021 nbsp 0183 32 The potential initial brand name drug that has the highest additional rebate calculated as a percentage of AMP should be used to determine the alternative rebate
Web 10 f 233 vr 2023 nbsp 0183 32 The statements included on this web page are intended to provide information on Medicaid Drug Rebate Program Releases and do not in any way revise Web 21 juil 2022 nbsp 0183 32 States must cover all covered outpatient drugs subject to a rebate agreement with the Secretary States must cover all covered outpatient drugs of a manufacturer that
Download Medicaid Rebate Agreement
More picture related to Medicaid Rebate Agreement

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN23MR18.008/original.png?1521650113

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN09NO16.006/original.png?1481698007

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN23MR18.006/original.png?1521650112
Web rebate which effectively anchors the Medicaid price of any drug to its in ation adjusted launch price In order to analyze how the MDRP affects drug prices we develop a Web 5 avr 2018 nbsp 0183 32 On March 23 CMS finalized updates to the Medicaid National Drug Rebate Agreement NDRA for the first time in 27 years to incorporate legislative and regulatory
Web Under the Medicaid Drug Rebate Program a drug manufacturer must enter into a Medicaid national drug rebate agreement with the Secretary of the U S Department of Health and Web The Medicaid Drug Rebate Program is a program in the United States that was created by the Omnibus Budget Reconciliation Act of 1990 OBRA 90 The program establishes

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN23MR18.009/original.png?1521650113

Federal Register Medicaid Program Announcement Of Medicaid Drug
https://s3.amazonaws.com/images.federalregister.gov/EN09NO16.011/original.png?1481698020

https://www.reedsmith.com/fr/perspectives/2023/06/proposed-medicaid...
Web 15 juin 2023 nbsp 0183 32 The Medicaid rebate statute 2 requires manufacturers of covered outpatient drugs to enter into a rebate agreement with CMS as a condition to federal funding for

https://www.medicaid.gov/.../medicaid-drug-rebate-program/index.html
Web 11 ao 251 t 2022 nbsp 0183 32 The program requires a drug manufacturer to enter into and have in effect a National Drug Rebate Agreement NDRA with the Secretary of the Department of

Federal Register Medicaid Program Announcement Of Medicaid Drug

Federal Register Medicaid Program Announcement Of Medicaid Drug

Federal Register Medicaid Program Announcement Of Medicaid Drug
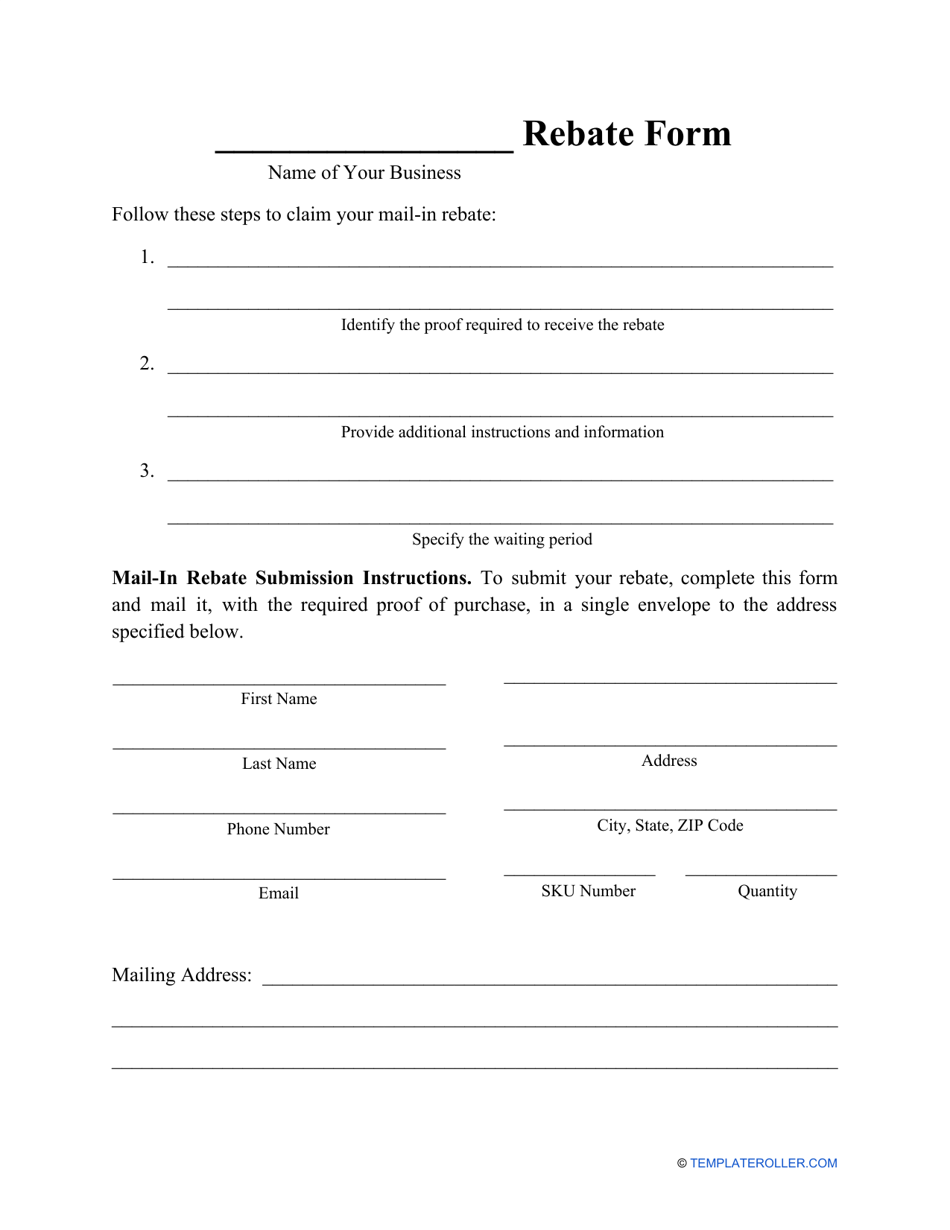
Supplier Rebate Agreement Template

TEXAS CHIP

Federal Register Medicaid Program Announcement Of Medicaid Drug

Federal Register Medicaid Program Announcement Of Medicaid Drug

Customer Rebate Agreement Template Master Template

Abbott Medicare Rebate Program Fill Online Printable Fillable

Florida Medicaid Provider Agreement Fill Online Printable Fillable
Medicaid Rebate Agreement - Web 8 janv 2021 nbsp 0183 32 The potential initial brand name drug that has the highest additional rebate calculated as a percentage of AMP should be used to determine the alternative rebate