What Is The Internal Energy Of An Object The internal energy of an object is intrinsically related to its temperature When a container containing gas molecules is heated up the molecules begin to move around faster increasing their kinetic energy
Changes in a material s temperature or state of matter are caused by changes to the internal energy The energy required by different materials depends on their heat capacity Internal energy in thermodynamics the property or state function that defines the energy of a substance in the absence of effects due to capillarity and external electric magnetic and other fields
What Is The Internal Energy Of An Object

What Is The Internal Energy Of An Object
https://themindpalace.in/wp-content/uploads/2020/10/Slide2-13.jpg

Science Chapter 11 Work And Energy Part 2
https://d1xuqjt1wg0fxw.cloudfront.net/f98ab6b0-290b-11ed-8846-6f83dbe265c6.jpg
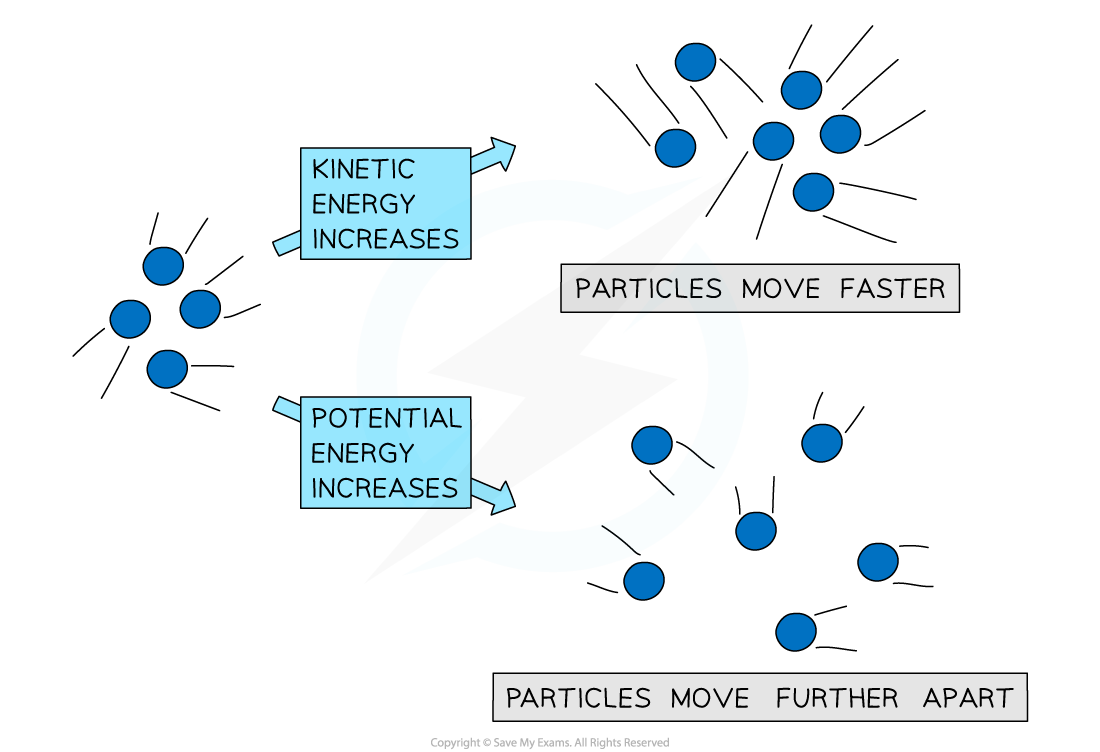
IB DP Physics SL 3 1 3 Internal Energy
https://oss.linstitute.net/wechatimg/2022/08/3-1-3-diagram-1-kinetic-energy-and-potential-energy-1.png
Internal Energy The internal energy E int of a thermodynamic system is by definition the sum of the mechanical energies of all the molecules or entities in the system The internal energy of a thermodynamic system is the energy of the system as a state function measured as the quantity of energy necessary to bring the system from its standard internal state to its present internal state of interest accounting for the gains and losses of energy due to changes in its internal state including such quantities
Internal energy is defined as the energy associated with the random disordered motion of molecules It is separated in scale from the macroscopic ordered energy associated with moving objects it refers to the invisible microscopic energy on the atomic and molecular scale Internal Energy The total energy stored inside a system by the particles that make up the system due to their motion and positions Molecules in a substance have kinetic energy since they are in motion and potential energy from their position relative to each other
Download What Is The Internal Energy Of An Object
More picture related to What Is The Internal Energy Of An Object

How To Find Kinetic Energy Joules Haiper
http://www.wikihow.com/images/f/f8/Calculate-Kinetic-Energy-Step-4-Version-2.jpg

The Internal Energy Of A System Is Expressed By Function U s V s
https://cdn.eduncle.com/library/scoop-files/2020/5/can_image_1589610425868.jpg
Solved Find Out The Internal Energy Of A System Which Has Chegg
https://media.cheggcdn.com/media/d2e/d2e723ef-747d-40f0-88a4-541f048bfa6f/phpzKvsGO
Internal energy of a body is a concept devised for bodies in thermodynamic equilibrium Its most important defining properties are It is a function of equilibrium state variables usually denoted as U Internal energy can be explained on a molecular level by taking into account the various terms that can contribute to this total energy This accounting process is easiest to carry out for a perfect gas sample because the particles do not interact with each other
[desc-10] [desc-11]

Edustood Study Material Internal Energy As A State Function
https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjFitho3SfdohLs5IsGKUNEUp-QPlfqUvey2O14cZMVtb51b0WYu9vImU7vYS0gPmi_8KovDdvk8P-3muNM7sQG7O0dXbXGuVu0orYiirUipR929goLfn0Xh0LD61d4a2Ax_FpbxPZx2pBDMp4YCvTTow3rAwdz3SJhL9ZGTtG0Gqu0E8np7tcFQyX8/s3072/illustrative image for solar energy finance.jpg

Spice Of Lyfe Physics Internal Energy Formula
https://i.ytimg.com/vi/k3rJs_ioTHc/maxresdefault.jpg

https://www.savemyexams.com/a-level/physics/cie/22...
The internal energy of an object is intrinsically related to its temperature When a container containing gas molecules is heated up the molecules begin to move around faster increasing their kinetic energy

https://www.bbc.co.uk/bitesize/guides/zcncjty/revision/2
Changes in a material s temperature or state of matter are caused by changes to the internal energy The energy required by different materials depends on their heat capacity

Biology Notes Part 36 Changing The Internal Energy Of A Material

Edustood Study Material Internal Energy As A State Function

Potential And Kinetic Energy Crossword Labs
Solved How To Define Exactly The Internal Energy 9to5Science

ENERGY Quizizz

What Is The Change In The Internal Energy Of A System Over One Complet

What Is The Change In The Internal Energy Of A System Over One Complet
SOLVED Which Of The Following Is True Of The Internal Energy Of A

SOLVED A System Gains A Certain Amount Of Energy In The Form Of Heat At

Forms Of Energy Save Energy
What Is The Internal Energy Of An Object - The internal energy of a thermodynamic system is the energy of the system as a state function measured as the quantity of energy necessary to bring the system from its standard internal state to its present internal state of interest accounting for the gains and losses of energy due to changes in its internal state including such quantities
