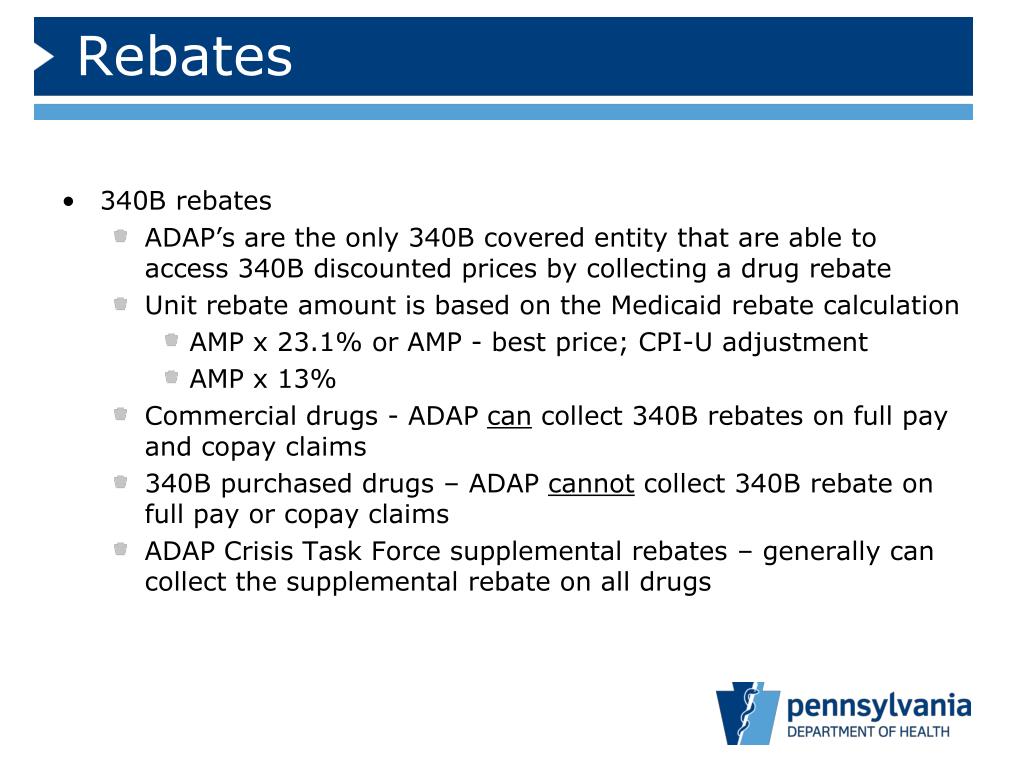
340b Rebates Congress created the Medicaid Drug Rebate Program in 1990 It required pharmaceutical manufacturers to provide rebates for medication purchases based on sales to Medicaid beneficiaries as a condition of having their products covered by Medicaid The amount of the rebates paid to the states were based on a quot best price quot calculation that did not take into account the discounted prices that manufacturers were offering directly to Federally funded clinics and p
Web 21 janv 2020 nbsp 0183 32 The 340B Drug Pricing Program 340B Program and the Medicaid Drug Rebate Program require manufacturers to provide discounts on outpatient drugs in Web The 340B ceiling price is the average manufacturer price AMP reduced by the unit rebate amount URA The URA is a minimum rebate percentage of 23 1 percent for most
340b Rebates

340b Rebates
https://assets.website-files.com/5e8b7c0fa2b4d57f247aa91e/62f3d3b786515cca2425acc0_Improve_Compliance_and_Create_Transparency-1-p-800.png
Introduction To 340B Rebates YouTube
https://i.ytimg.com/vi/_QDOKr4vCOI/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-DoACuAiKAgwIABABGEYgVyhyMA8=&rs=AOn4CLDwT4DNGm4_tu7z2G4Uf0n6DN2xtg
CMS Seeks Input On Excluding 340B Drugs From New Medicare Part D
https://340breport.com/wp-content/uploads/2023/02/shutterstock_image100-940x510.jpg
Web The 340B ceiling price is the maximum statutory price a manufacturer can charge a covered entity for the purchase of a covered outpatient drug and is equal to the average Web 14 janv 2022 nbsp 0183 32 The 340B rebate model operationalized at contract pharmacies is designed to support the needs of all stakeholders in 340B covered entities and their contract
Web 11 mai 2023 nbsp 0183 32 The 340B Program enables covered entities to stretch scarce federal resources as far as possible reaching more eligible patients and providing more Web 11 mai 2023 nbsp 0183 32 What GAO Found The 340B Drug Pricing Program 340B Program requires drug manufacturers to sell outpatient drugs at discounted prices to covered entities
Download 340b Rebates
More picture related to 340b Rebates
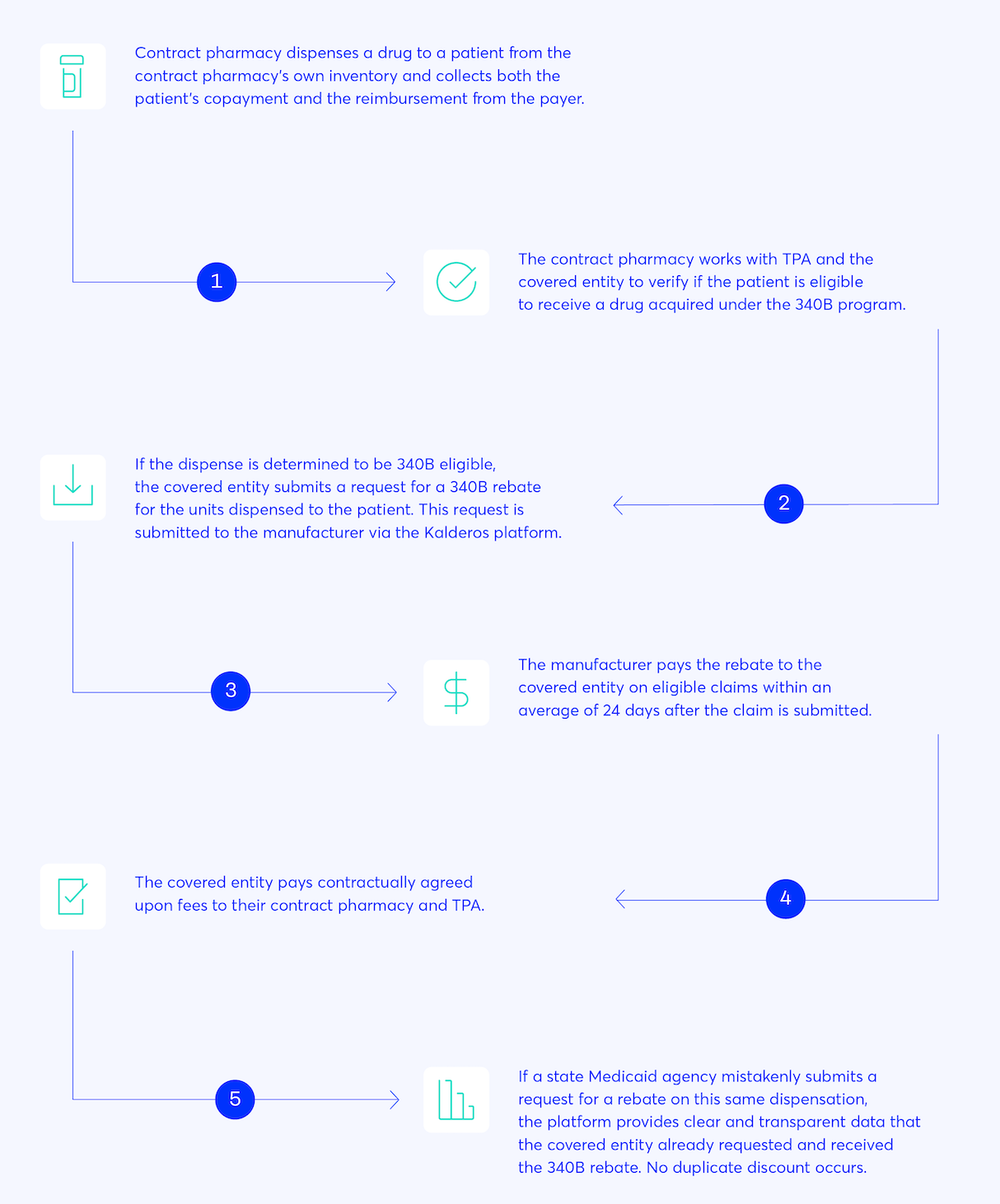
Cash Flow Analysis Of The 340B Rebate Model 3 XIS Advisors
https://images.squarespace-cdn.com/content/v1/5c326d5596e76f58ee234632/8a51c499-50e8-4a99-9a79-fbd66819aa81/340_rebate_model_in_action_3axis_blog_image.png

PPT The Federal 340B Drug Discount Program A Primer PowerPoint
https://image.slideserve.com/1284887/340b-pharmacy-arrangements-l.jpg

News VativoRx Pharmacy Benefits Management
https://vativorx.com/wp-content/uploads/2023/05/340B-Post-768x514.jpg
Web 10 sept 2021 nbsp 0183 32 Learn about the history of 340BRebates and how a rebate solution for 340B discounts benefits manufacturers and providers by drastically reducing the risk Web 1 nov 2021 nbsp 0183 32 The Kalderos 340B rebate model represents a unifying solution to the current challenges and controversies surrounding contract pharmacy participation in 340B The rebate model operationalized at
Web About the Coalition We are a broad coalition of providers and programs representing the thousands of hospitals community health centers and clinics in support of protecting Web This guidance is issued in accordance with section 1847A c 5 C of the Social Security Act the Act and is directed to Medicare providers and suppliers who bill for separately

Kalderos 340B Rebate Model Improves Covered Entity Cash Flow Study By
https://assets.website-files.com/5e8b7c0fa2b4d57f247aa91e/62f12faeedb2096d3c02796c_62e1930a7b467a55ffdaa5da_3_Axis_Study_1%25402x.png

Recent Changes To The 340B Drug Pricing Program
https://blog.definitivehc.com/hs-fs/hubfs/Imported_Blog_Media/hc-735x1024-2.png?width=984&height=1371&name=hc-735x1024-2.png

https://en.wikipedia.org/wiki/340B_Drug_Pricing_Program
Congress created the Medicaid Drug Rebate Program in 1990 It required pharmaceutical manufacturers to provide rebates for medication purchases based on sales to Medicaid beneficiaries as a condition of having their products covered by Medicaid The amount of the rebates paid to the states were based on a quot best price quot calculation that did not take into account the discounted prices that manufacturers were offering directly to Federally funded clinics and p

https://www.gao.gov/products/gao-20-212
Web 21 janv 2020 nbsp 0183 32 The 340B Drug Pricing Program 340B Program and the Medicaid Drug Rebate Program require manufacturers to provide discounts on outpatient drugs in
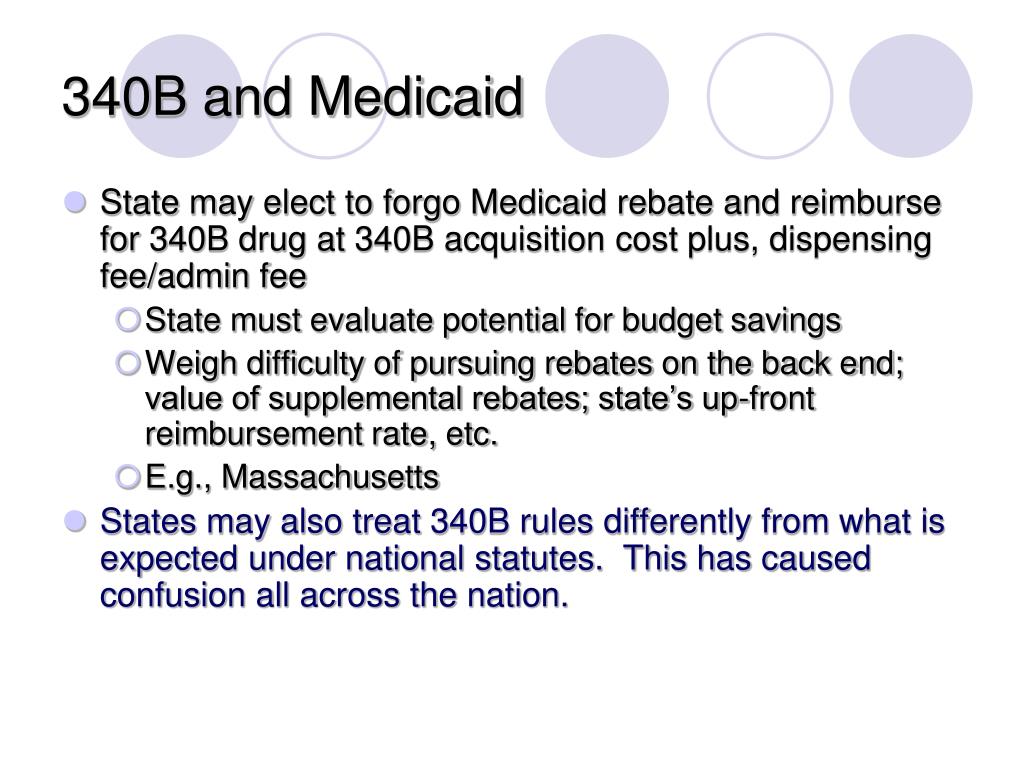
PPT 340B An Overview PowerPoint Presentation Free Download ID 4747618

Kalderos 340B Rebate Model Improves Covered Entity Cash Flow Study By

HOME ASAP 340B

The Kalderos 340B Rebate Model In Action
PPT Pennsylvania ADAP 340B PowerPoint Presentation Free Download
BREAKING Bipartisan Group Of 217 U S Representatives Ask HHS To Stop

BREAKING Bipartisan Group Of 217 U S Representatives Ask HHS To Stop

Understanding The Mechanics Of The 340B Drug Pricing Program HENRY KOTULA

PPT The 340B Program An Overview PowerPoint Presentation Free

Kalderos Tells Hospitals Its Service To Turn 340B Discounts Into
340b Rebates - Web 14 janv 2022 nbsp 0183 32 The 340B rebate model operationalized at contract pharmacies is designed to support the needs of all stakeholders in 340B covered entities and their contract